All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Management of patients with PsA following inadequate response or intolerance to TNF inhibitors
Do you know... Which of the following FDA-approved treatments for PsA is a dual IL-17A and IL-17F inhibitor, with efficacy in patients who have experienced an inadequate response to TNF inhibitors?
Introduction
Psoriatic arthritis (PsA) is a chronic, heterogeneous, immune-mediated inflammatory disease affecting 20–30% of patients with plaque psoriasis (PsO). It is characterized by peripheral arthritis, enthesitis, dactylitis, axial inflammation, and psoriasis of skin and nails.1,2 When conventional disease-modifying antirheumatic drugs (DMARDs) prove inadequate for managing PsA, biological DMARDs, most commonly tumor necrosis factor α inhibitors (TNFi), are prescribed.3 However, a considerable proportion of patients experience inadequate response or intolerance to TNFis.3 Moreover, when the initial TNFi fails to demonstrate clinical efficacy, subsequent TNFi therapies are generally associated with diminished response rates.2 Consequently, there is a need for therapies with alternative mechanisms of action, and proven safety and efficacy, in this population.3
Below, we summarize available therapeutic options for patients with PsA who have experienced an inadequate response to TNFi (TNFi-IR).
Alternate biologic DMARDs: Interleukin inhibitors
Interleukin (IL)-23 and -17 cytokines have emerged as key drivers in the pathogenesis of PsO and PsA.4 Several agents directed against IL-23 and IL-17 pathways are approved for PsA, and have shown efficacy and acceptable safety profiles in patients with TNFi-IR.
Secukinumab
Secukinumab is a fully human monoclonal antibody (mAb) that selectively targets IL-17A and is approved for the treatment of PsO and PsA. In the four phase III FUTURE trials,5–8 which included both TNFi-naïve and TNFi-IR populations, secukinumab demonstrated improvements in signs and symptoms of PsA in patients with TNFi-IR (Table 1). However, a systematic review and meta-analysis of the phase II FUTURE 1 and phase III FUTURE 2, 3, and 5 trials revealed that a significantly smaller proportion of patients with TNFi-IR achieved ≥20% improvement from baseline in American College of Rheumatology response criteria (ACR20) compared with patients naïve to TNFi (risk ratio [RR], 0.71; p < 0.001).4 Furthermore, a post hoc analysis of the FUTURE 2–5 trials indicated that response rates across GRAPPA-OMERACT PsA core domains were higher in TNFi-naïve patients than in those with prior TNFi exposure.9 These findings suggest that while secukinumab remains effective in TNFi-IR populations, its clinical benefit may be more pronounced when used earlier in the treatment sequencing, potentially as a first-line biologic agent in PsA.
Table 1. Efficacy of treatments in patients with PsA and TNF-IR*
Treatment | Trial | Primary ACR response endpoint |
Interleukin inhibitors | ||
Secukinumab
| FUTURE 2 (NCT01752634)5 | ACR20 at Week 24 Secukinumab 300 mg SC: 45% (n = 33; p = 0.0077) Secukinumab 150 mg SC: 30% (n = 37; p = 0.1216) Placebo: 14% (n = 35) |
FUTURE 3 (NCT01989468)6 | ACR20 at Week 24 (n = 132) Secukinumab 300 mg autoinjector: 40.9% (p < 0.01) Secukinumab 150 mg autoinjector: 34.1% (p < 0.05) Placebo: 9.1% | |
FUTURE 4 (NCT02294227)7 | ACR20 at Week 16 (n = 27) Secukinumab 150 mg SC no load: 25.9% Placebo: 11.1% | |
FUTURE 5 (NCT02404350)8 | ACR20 at Week 16 Secukinumab 300 mg SC: 51.5% (n = 68; p < 0.0001) Secukinumab 150 mg SC: 50.8% (n = 65; p < 0.0001) Secukinumab 150 mg SC no load: 39.1% (n = 64; p < 0.01) Placebo: 18.4% (n = 98) | |
Bimekizumab | BE COMPLETE (NCT03896581)1 | ACR50 at Week 16 (N = 400) Placebo: 6.8% |
Guselkumab | COSMOS (NCT03796858)10 | ACR20 at Week 24 (N = 285) Guselkumab 100 mg Q8W: 44.4% (p < 0.001) Placebo: 19.8% |
Ixekizumab | SPIRIT-P2 (NCT02349295)3 | ACR20 at Week 24 (N = 363) Ixekizumab 80 mg SC Q4W: 53% (p < 0.0001) Ixekizumab 160 mg SC Q2W: 48% (p < 0.0001) Placebo: 19% |
JAK inhibitors | ||
Tofacitinib | OPAL Beyond (NCT01882439)11 | ACR20 at 3 months (N = 395) Tofacitinib 5 mg oral: 50% (p < 0.001) Tofacitinib 10 mg oral: 47% (p < 0.001) Placebo: 24% |
ACR, American College of Rheumatology; ACR20/50, ≥20%/≥50% improvement from baseline in American College of Rheumatology response criteria; HAQ-DI, Health Assessment Questionnaire–Disability Index; JAK, Janus kinase; Q2W, every 2 weeks; Q4W, every 4 weeks; SC, subcutaneous *Data from Merola et al.,1 Nash et al.,3 McInnes et al.,5 Nash et al.,6 Kivitz et al.,7 Mease et al.,8 Coates et al.,10 and Gladman et al.11 | ||
Bimekizumab
Bimekizumab is a monoclonal immunoglobulin G1 (IgG1) antibody and is the only IL-17A and IL-17F dual inhibitor approved for the treatment of adult patients with PsO and PsA. Its approval in PsA was supported by data from the phase III BE COMPLETE (NCT03896581) trial, conducted exclusively in patients with active PsA and prior TNFi-IR, in which bimekizumab showed higher response rates vs placebo across joint and skin outcomes at Week 16 (Table 1).1
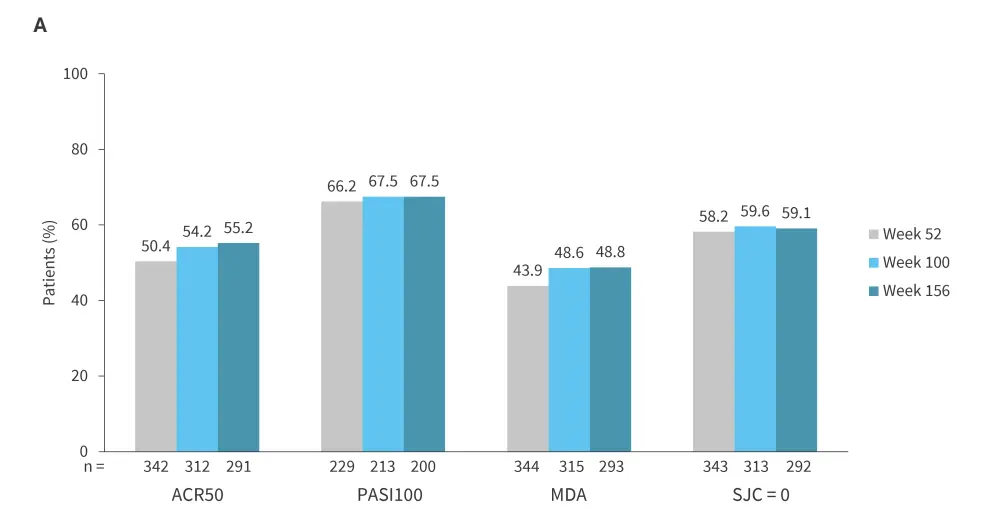
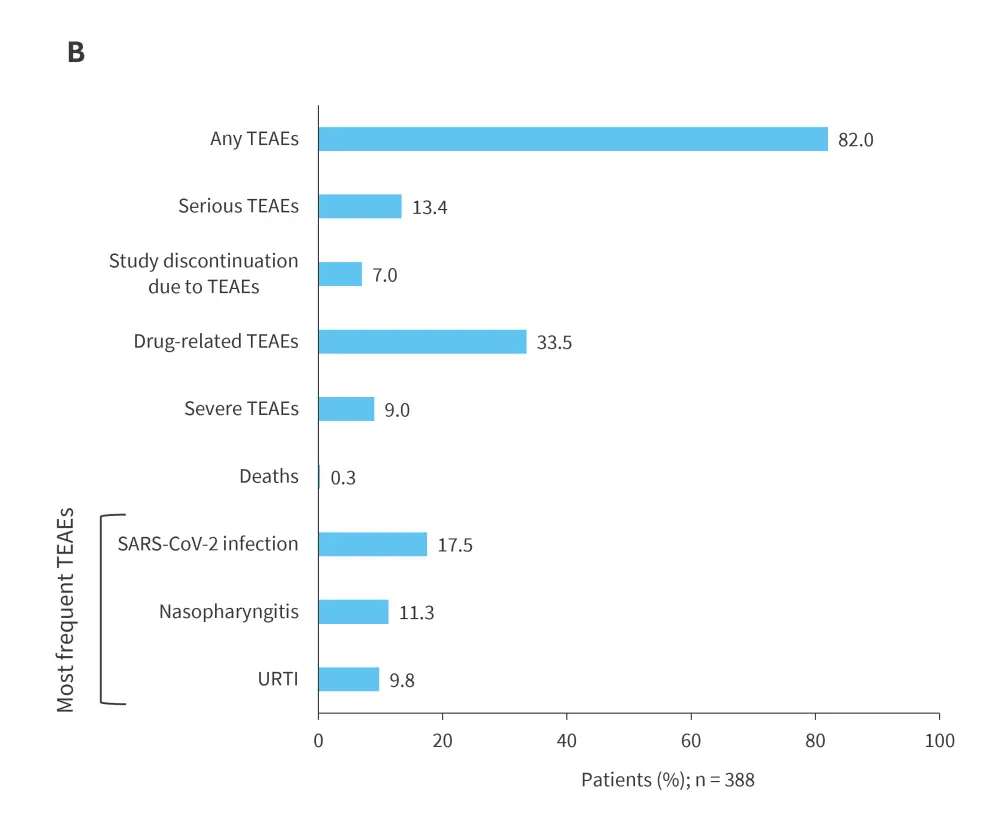
Patients who completed the 16-week BE COMPLETE trial subsequently entered the open-label extension study, BE VITAL (NCT04009499), in which those who previously received placebo were transitioned to bimekizumab. Sustained efficacy was observed from Year 1 through Year 3 across all assessed endpoints, including ≥50% improvement from baseline in American College of Rheumatology response criteria (ACR50), complete skin clearance measured by ≥100% improvement from baseline in Psoriasis Area and Severity Index (PASI100), minimal disease activity (MDA), and resolution of swollen joints (SJC = 0; Figure 1A).12 Bimekizumab was well tolerated over the extended treatment period, with no new safety signals identified (Figure 1B); Candida accounted for the majority of infections, which were largely mild/moderate in severity, with a low incidence of treatment discontinuation.12 Collectively, results from the BE COMPLETE and BE VITAL studies demonstrate that bimekizumab provides sustained and high levels of response for up to 3 years in patients with TNFi-IR PsA.12
Figure 1. Long-term A efficacy and B tolerability over time to Week 156 in BE COMPLETE and BE VITAL OLE (mNRI)*
Guselkumab
Guselkumab is a fully human IL−23 p19-subunit inhibitor approved for the treatment of PsO and PsA. Its efficacy and safety in patients with PsA and TNF-IR were evaluated in the phase III COSMOS trial (NCT03796858).10 At Week 24, guselkumab demonstrated significant improvements in signs and symptoms of PsA compared with placebo (Table 1),10 consistent across diverse baseline-defined subgroups of patients with TNFi-IR PsA.13 Further, a post hoc analysis showed that guselkumab provided substantial real-world benefits in this population across composite indices, including both patient-reported and physician-assessed outcome measures.14
Ixekizumab
Ixekizumab is a monoclonal antibody that selectively targets IL-17A and is approved for the treatment of PsO and PsA. The phase III SPIRIT-P2 trial (NCT02349295) evaluated the efficacy and safety of ixekizumab in patients with active PsA and TNFi-IR. At Week 24, ixekizumab demonstrated improvements in clinical signs and symptoms of PsA compared with placebo (Table 1).3
Patients completing the 24-week double-blind period entered a long-term extension period (Weeks 24–156; n = 310), during which those who previously received placebo were re-randomized to receive ixekizumab every 4 weeks (Q4W) and every 2 weeks (Q2W; 1:1).2 Clinical responses were maintained through Week 156, with ACR20 response rates of 84% and 85% in the Q4W and Q2W groups, respectively.2 These results support the long-term efficacy and durability of ixekizumab as a treatment option for patients with PsA with TNFi-IR.2
Targeted synthetic DMARDs: JAK inhibitors
Tofacitinib
Tofacitinib is an oral Janus kinase (JAK) inhibitor evaluated for the treatment of PsA in the phase III OPAL Beyond trial (NCT01882439), which included patients with active PsA and TNFi-IR. At 3 months, tofacitinib demonstrated significantly greater efficacy than placebo in both co-primary endpoints: ACR20 and Health Assessment Questionnaire–Disability Index (HAQ-DI) scores (Table 1).11 These findings led to its approval in PsA and support its use as an effective treatment option for patients with PsA following TNFi failure.11
Conclusions
Patients with PsA who exhibit TNFi-IR are a challenging subgroup, particularly as second-line biologic treatments are often associated with reduced clinical efficacy.15 Emerging agents targeting the IL-17 pathway – such as secukinumab, bimekizumab, and ixekizumab – as well as those targeting the IL-23 pathway, including guselkumab, have demonstrated significant improvements in both joint and skin symptoms in these patients. While secukinumab has shown efficacy in TNFi-IR PsA, its benefits appear less pronounced than when used as a first-line biologic; in contrast, bimekizumab’s dual inhibition of IL-17A and IL-17F has resulted in robust clinical responses in this patient population. Additionally, the oral JAK inhibitor tofacitinib has proven effective in TNFi-IR cases and offers the convenience of a non-injectable option. Among available treatments, bimekizumab and ixekizumab currently have the most robust long-term efficacy and tolerability data for over 3 years. Collectively, these therapeutic advances are broadening the treatment landscape for patients with TNFi-IR, supporting a more personalized and evidence-based approach to optimizing long-term management in PsA.
This educational resource is independently supported by UCB. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?


