All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Bimekizumab for patients with PsA: Results from the BE OPTIMAL and BE COMPLETE trials
During the American College of Rheumatology (ACR) annual meeting (ACR Convergence 2022), results were presented from two phase III trials of bimekizumab in patients with psoriatic arthritis (PsA). The BE OPTIMAL trial (NCT03895203), investigating the efficacy of bimekizumab in patients who were biologic DMARD (disease-modifying antirheumatic drug)-naïve, was presented by Ritchlin; the BE COMPLETE trial (NCT03896581), assessing bimekizumab in patients who did not respond to previous treatment with tumor necrosis factor inhibitors (TNFis) or were intolerant, was presented by Merola.1,2
Here, we summarize key data from these two trials. More information on the mode of action of bimekizumab and recent approval data has been previously reported by the Psoriasis and Psoriatic Arthritis Hub.
BE OPTIMAL
Study design
The BE OPTIMAL study enrolled 852 patients and was conducted over a 52-week period. Adalimumab was used as the reference arm for this trial, with patients in the placebo arm receiving open-label bimekizumab from Week 16 to 52. The study design is outlined in Figure 1.
Figure 1. BE OPTIMAL study design and inclusion criteria*
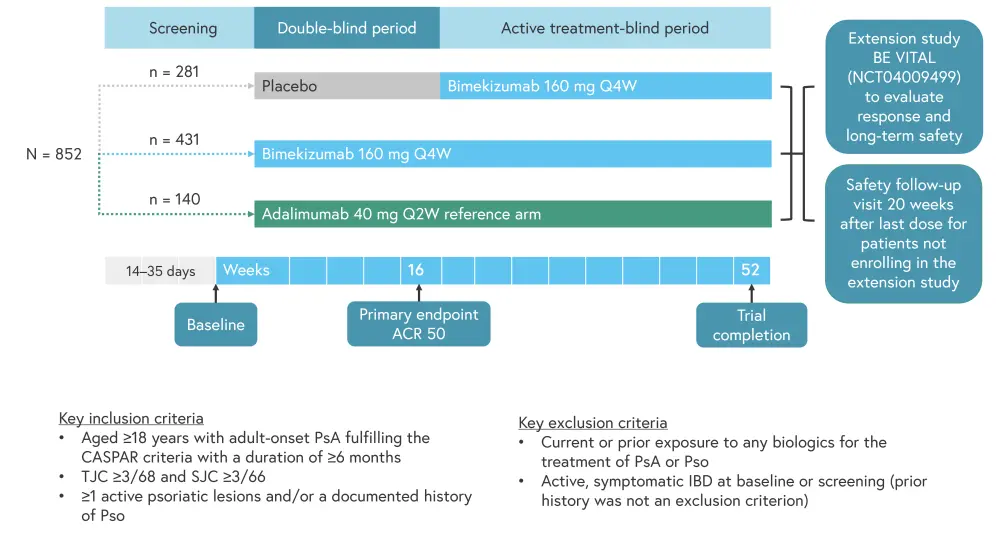
ACR50, American College of Rheumatology response criteria; CASPAR, classification Criteria for psoriatic arthritis; IBD, irritable bowel disease; Q2W, every 2 weeks; Q4W, every 4 weeks; PsA, psoriatic arthritis; Pso, psoriasis; SJC, swollen joint count; TJC, tender joint count.
*Adapted from Ritchlin.1
Baseline patient characteristics are displayed in Table 1. Patient characteristics were mostly well balanced between the arms; however, the prevalence of dactylitis and enthesitis was slightly higher in the bimekizumab arm compared with the other two groups. Almost 50% of all patients had psoriasis over ≥3% of their body surface area.
Table 1. Baseline characteristics*
|
Ada, adalimumab; BMI, body mass index; BSA, body surface area; HAQ-DI, Health Assessment Questionnaire-Disability Index; hs-CRP, high-sensitivity c-reactive protein; PCS, Physical Component Summary; PsA, psoriatic arthritis; Pso, psoriasis; Q2W, every 2 weeks; Q4W, every 4 weeks; SD, standard deviation; SF-36, Short-Form 36-item Health Survey; SJC, swollen joint count; TJC, tender joint count; vdHm TSS, van der Heijde modified total Sharp score. |
|||
|
Characteristic |
Placebo (n = 281) |
Bimekizumab 160 mg |
Ada 40 mg Q2W |
|---|---|---|---|
|
Mean age (SD), years |
48.7 (11.7) |
48.5 (12.6) |
49.0 (12.8) |
|
Male, % |
45.2 |
46.6 |
50.7 |
|
Mean BMI (SD), kg/m2 |
29.6 (6.1) |
29.2 (6.8) |
28.4 (5.9) |
|
Mean PsA duration |
5.6 (6.5) |
6.0 (7.3) |
6.1 (6.8) |
|
Concomitant |
58.0 |
58.5 |
58.6 |
|
Mean TJC of 68 joints (SD), n |
17.1 (12.5) |
16.8 (11.8) |
17.5 (13.1) |
|
Mean SJC of 68 joints (SD), n |
9.5 (7.3) |
9.0 (6.2) |
9.6 (7.1) |
|
hs-CRP ≥6 mg/L, % |
43.1 |
36.7 |
31.4 |
|
Psoriasis BSA ≥3%, % |
49.8 |
50.3 |
48.6 |
|
Mean PASI score (SD), |
7.9 (5.6) |
8.2 (6.8) |
8.5 (7.6) |
|
Mean HAQ-DI (SD), n |
0.89 (0.61) |
0.82 (0.59) |
0.86 (0.54) |
|
Mean SF-36 PCS (SD), |
36.9 (9.7) |
38.1 (9.4) |
37.6 (8.8) |
|
Mean vdHm TSS (SD), |
|
|
|
|
At-risk |
14.5 (23.9) |
14.4 (32.0) |
16.5 (28.4) |
|
Overall |
12.3 (22.5) |
12.5 (30.0) |
13.8 (26.5) |
|
Enthesitis, % |
24.9 |
33.2 |
25.7 |
|
Mean Score |
2.9 (1.5) |
2.5 (13.0) |
2.3 (1.6) |
|
Dactylitis, % |
11.7 |
13.0 |
7.9 |
|
Mean Score |
47.3 (41.1) |
46.7 (54.3) |
49.7 (31.9) |
Overall, 89.3% of patients enrolled completed week 52 of treatment. Adverse events (AEs) or withdrawal of consent were the main reasons for discontinuation of treatment once patients were randomized on the study (Figure 2).
Figure 2. Patient disposition*
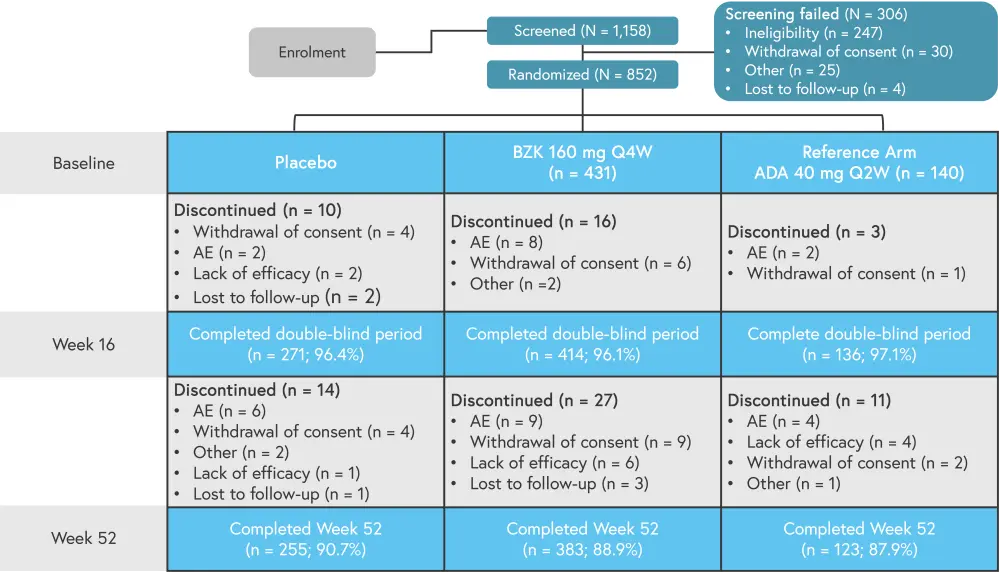
AE, adverse event; Q2W, every 2 weeks; Q4W, every 4 weeks; TEAE, treatment-emergent AEs.
*Adapted from Ritchlin.1
The primary endpoint was ACR 50 response at Week 16 (Figure 3A). The bimekizumab arm showed a significant increase in ACR 50 compared with placebo at Week 16 (p < 0.001) and had a similar effect compared with the reference arm. In addition, bimekizumab was shown to be an effective salvage therapy for patients in the placebo arm during the active-treatment blind period.
Secondary endpoints included Psoriasis Area and Severity Index (PASI)100, which was significantly increased in the bimekizumab arm compared with placebo at Week 16 (p < 0.001; Figure 3B). A lower percentage of patients in the reference arm achieved a PASI100 score at Week 16 compared with the bimekizmab arm (20.6% vs 47.5%); however, this disparity was less evident by Week 52 (60.8% vs 48.5%).
Radiographic progression of disease was inhibited in all groups at all time points in >87% of patients (Figure 3C).
Figure 3. A ACR 50, B PASI100, and C radiographic progression of enrolled patients*
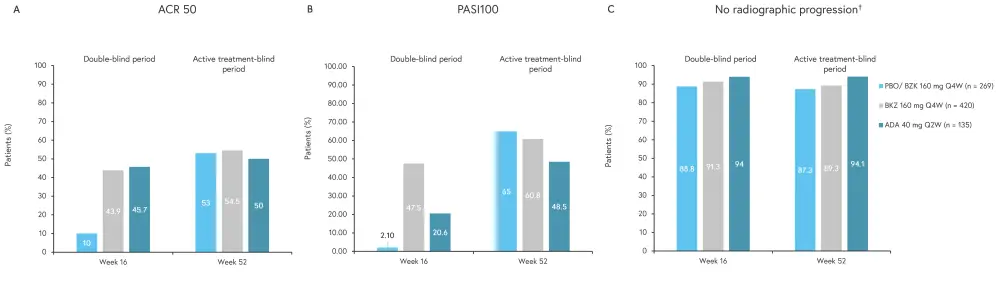
ACR 50, American College of Rheumatology Criteria ≥50% response; Ada, adalimumab; BZK, bimekizumab; PASI100, 100% improvement in Psoriasis Area and Severity Index; PBO, placebo; Q2W, every 2 weeks; Q4W, every 4 weeks; vdHm TSS, van der Heijde modified total Sharp score.
*Adapted from Ritchlin.1
†vdHm TSS change from baseline ≤0.5.
Minimal disease activity (MDA; Figure 4) was significantly increased in the bimekizumab arm compared with placebo at Week 16 (45.0% vs 13.2%; p < 0.001). At Week 52, patients achieving MDA significantly increased to 53.7% in the placebo/bimekizumab arm compared with 52.9% and 55% in the adalimumab arm and bimekizumab arm, respectively.
Figure 4. MDA response criteria*
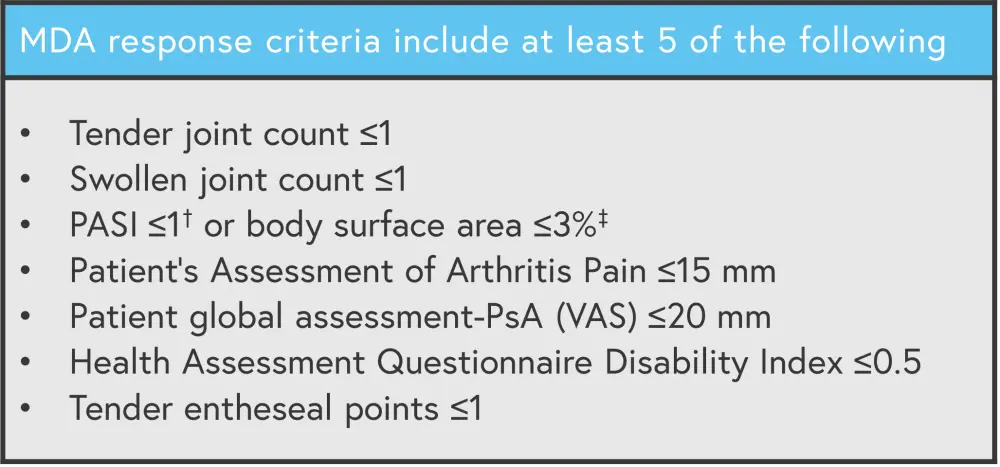
MDA, minimal disease activity; VAS, visual analogue scale.
*Adapted from Ritchlin.1
†For patients with Pso involving ≥3% of BSA at baseline.
‡Patients with Pso involving ≤3% of BSA at baseline will always meet the criteria PASI ≤1 or BSA ≤3%, except in cases where a BSA >3% is observed.
Safety
Overall, more patients experienced treatment-emergent AEs (TEAEs) the two treatment arms compared with placebo from baseline to Week 16; however, there was only a small difference in the number of serious TEAEs between groups (Table 2). The most common TEAEs were nasopharyngitis, upper respiratory tract infection, and urinary tract infection. Only one death occurred on the study, which was due to a motorcycle accident.
Table 2. Safety from Week 0–52*
|
Ada, adalimumab; ALT, alanine aminotransferase; AST, aspartate transaminase; BZK, bimekizumab; IBD, irritable bowel disease; MACE, major adverse cardiovascular event; Q2W, every 2 weeks; Q4W, every 4 weeks; TEAE, treatment-emergent adverse events; UTI, urinary tract infection; URTI, upper respiratory tract infection. |
|||||
|
TEAE, % (unless stated |
Week 0−16 |
Week 0−52 |
|||
|---|---|---|---|---|---|
|
Placebo |
BZK 160 mg |
Ada 40 mg |
BKZ 160 mg |
Ada 40 mg |
|
|
Any TEAE |
49.5 |
59.6 |
59.3 |
79.1 |
80.7 |
|
Serious TEAEs |
1.1 |
1.9 |
1.4 |
6.6 |
7.1 |
|
TEAEs leading to discontinuation |
1.1 |
1.9 |
2.1 |
3.0 |
5.0 |
|
Drug-related TEAEs |
12.5 |
23.2 |
24.3 |
31.9 |
38.6 |
|
Severe TEAEs |
0 |
0.9 |
2.1 |
3.3 |
6.4 |
|
Deaths, n |
0 |
0 |
0 |
1‡ |
0 |
|
Most frequent TEAEs (≥5%) |
|||||
|
Nasopharyngitis |
4.6 |
9.3 |
5.0 |
12.0 |
8.6 |
|
URTI |
6.4 |
5.1 |
2.1 |
7.1 |
5.7 |
|
UTI |
1.4 |
2.1 |
2.1 |
6.1 |
3.6 |
|
Headache |
2.5 |
4.4 |
1.4 |
5.8 |
4.3 |
|
Oral candidiasis§ |
0 |
2.1 |
0 |
5.4 |
0.7 |
|
Diarrhea |
2.5 |
3.7 |
3.6 |
5.1 |
5.0 |
|
Hypertension |
3.9 |
2.8 |
2.9 |
4.1 |
6.4 |
|
ALT elevation |
0.7 |
0.7 |
5.0 |
2.3 |
7.9 |
|
AST elevation |
0.7 |
0.2 |
2.9 |
2.0 |
5.0 |
|
Injection-site erythema |
0 |
0.2 |
2.9 |
0.9 |
5.0 |
|
TEAEs of special interest |
|||||
|
Candidiasis§ |
0.7 |
2.6 |
0 |
7.7 |
0.7 |
|
Serious infections |
0 |
0.2 |
0.7 |
0.9 |
1.4 |
|
Adjudicated MACE |
0 |
0 |
0 |
0.8 |
1.4 |
|
Definite adjudicated IBD |
0 |
0 |
0 |
0.3 |
0 |
|
Malignancies |
0.4 |
0.2 |
0 |
1.0 |
0 |
|
Non |
0 |
0.2 |
0 |
0.6 |
0 |
BE COMPLETE
Results from the phase III BE COMPLETE trial, investigating bimekizumab in patients with active PsA (N = 400) and prior inadequate response or intolerance to tumor necrosis factor inhibitors (TNFis), were presented by Merola.2 The results from the BE COMPLETE trial have also been published in a recent article in the Lancet.3 Patients were split 2:1 between the bimekizumab and placebo arms, respectively (Figure 5).
Figure 5. BE COMPLETE study design*
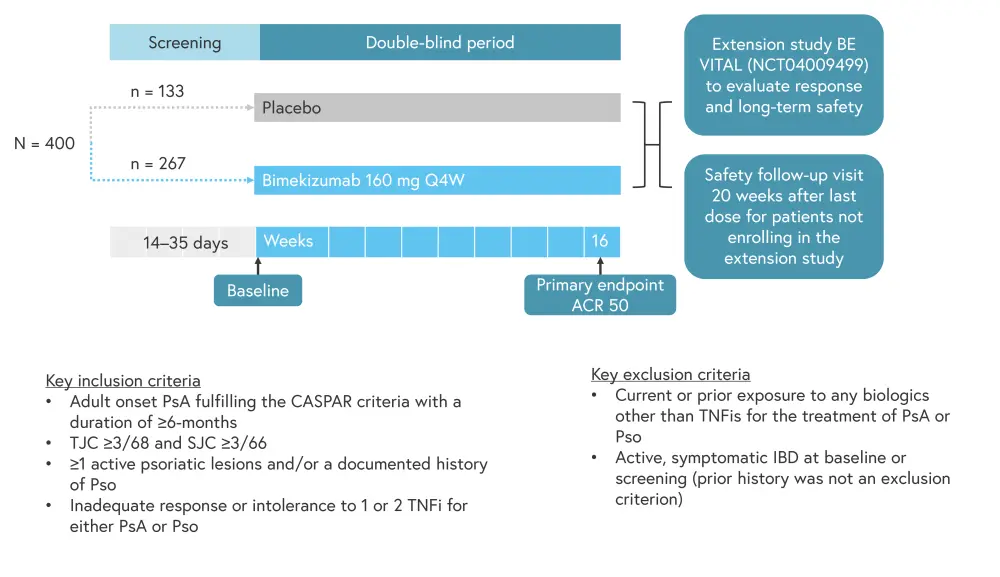
ACR50, American College of Rheumatology Criteria ≥50% response; CASPAR, classification criteria for psoriatic arthritis; IBD, irritable bowel disease; Q4W, every 4 weeks; PsA, psoriatic arthritis; Pso, psoriasis; SJC, swollen joint count; TJC, tender joint count; TNFi, tumor necrosis factor inhibitor.
*Adapted from Merola.2
Patient characteristics were well balanced between arms (Table 3); however, concomitant methotrexate use was higher in the bimekizumab arm compared with the placebo arm (44.6% vs 38.3%). In both groups, >10% of patients had inadequate response to two TNFis and >11% were intolerant to TNFis (patients who were unable to access TNFis were not included in these groups).
Table 3. BE COMPLETE patient characteristics*
|
Ada, adalimumab; BMI, body mass index; BSA, body surface area; HAQ-DI, Health Assessment Questionnaire-Disability Index; hs-CRP, high-sensitivity c-reactive protein; LDI, Leeds Dactylitis Index; LEI, Leeds Enthesitis Index; mNAPSI, modified Nail Psoriasis Severity Index; PASI, Psoriasis Area and Severity Index; PCS, Physical Component Summary; PsA, psoriatic arthritis; Pso, psoriasis; PtAAP, Patient’s Assessment of Arthritis Pain; Q4W, every 4 weeks; SD, standard deviation; SF-36, Short-Form 36-item Health Survey; SJC, swollen joint count; TJC, tender joint count; TNFi, tumor necrosis factor inhibitor. |
||
|
Characteristic |
Placebo (n = 133) |
Bimekizumab |
|---|---|---|
|
Mean age (SD), years |
51.3 (12.9) |
50.1 (12.4) |
|
Male, % |
45.1 |
48.7 |
|
Mean BMI (SD), kg/m2 |
29.0 (5.4) |
30.1 (6.5) |
|
Mean PsA duration (SD), years |
9.2 (8.1) |
9.6 (9.9) |
|
Concomitant methotrexate, % |
38.3 |
44.6 |
|
Prior TNFi exposure, % |
|
|
|
Inadequate response to 1 TNFi |
77.4 |
76.4 |
|
Inadequate response to 2 TNFis |
11.3 |
10.9 |
|
Intolerance to TNFi |
11.3 |
12.7 |
|
Mean TJC of 68 joints (SD), n |
19.3 (14.2) |
16.8 (13.5) |
|
Mean SJC of 68 joints (SD), n |
10.3 (8.2) |
9.7 (6.2) |
|
hs-CRP ≥6 mg/L, % |
44.4 |
44.2 |
|
Psoriasis BSA ≥3%, % |
66.2 |
65.9 |
|
Mean PASI score (SD), n |
8.5 (6.6) |
10.1 (9.1) |
|
Mean HAQ-DI (SD), n |
1.04 (0.69) |
0.97 (0.59) |
|
Mean PtAAP (SD), n |
61.7 (24.6) |
58.3 (24.2) |
|
Mean SF-36 PCS (SD), n |
35.9 (10.2) |
36.4 (9.0) |
|
Nail psoriasis, % |
62.4 |
59.6 |
|
Mean mNAPSI score (SD), n |
4.5 (2.8) |
4.3 (2.8) |
|
Enthesitis, % |
27.1 |
39.7 |
|
Mean LEI score (SD), n |
2.9 (1.6) |
2.6 (1.5) |
|
Dactylitis, % |
10.5 |
12.7 |
|
Mean LDI score (SD), n |
66.4 (127.6) |
72.7 (114.4) |
The primary endpoint was ACR 50 and secondary endpoints included PASI90, both showed a significant increase in the bimekizumab arm compared with placebo at Week 16 (p < 0.001; Figure 6).
Figure 6. ACR 50 and PASI100 results*
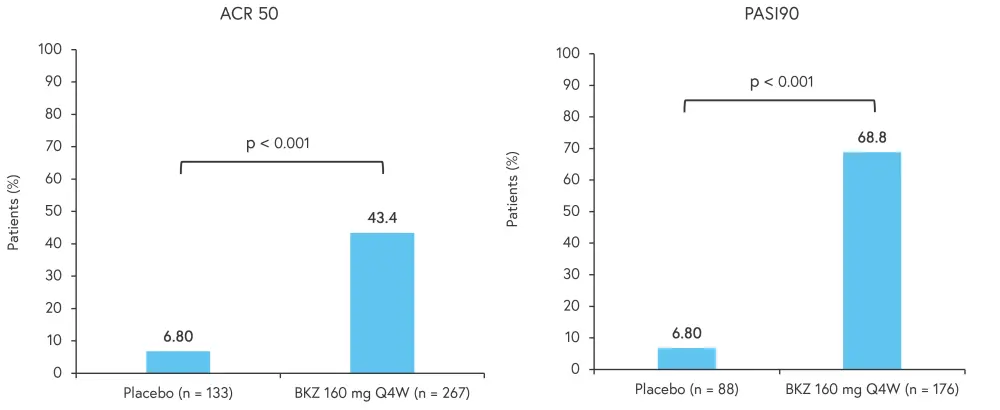
ACR 50, American College of Rheumatology Criteria ≥50% response; Ada, adalimumab; BZK, bimekizumab; PASI90, 90% improvement in Psoriasis Area and Severity Index; PBO, placebo; Q2W, every 2 weeks; Q4W, every 4 weeks.
*Adapted from Merola.2
Improvements were seen in the bimekizumab arm compared with the placebo arm for all secondary endpoints (Table 4).
Table 4. Secondary endpoints at Week 16*
|
ACR50, American College of Rheumatology Criteria ≥50% response; ACR70, ACR criteria ≥70% response; HAQ-DI, Health Assessment Questionnaire-Disability Index; MDA, minimal disease activity; mNAPSI, modified nail psoriasis severity index; PASI90, 90% improvement in Psoriasis Area and Severity Index; PASI100, 100% improvement in PASI; PCS, Physical Component Summary; Q2W, every 2 weeks; Q4W, every 4 weeks; SF-36, Short-Form 36-item Health Survey; SJC, swollen joint count; TJC, tender joint count. |
|||
|
Endpoint, % (unless |
Placebo |
BZK 160 mg |
p value |
|---|---|---|---|
|
ACR 70 |
0.8 |
26.6 |
<0.001 |
|
PASI90 |
4.5 |
58.5 |
<0.001 |
|
MDA |
6.0 |
44.2 |
<0.001 |
|
ACR50 + PASI100 |
1.1 |
33.5 |
<0.001 |
|
TJC change from baseline |
−2.4 |
−10.9 |
— |
|
SJC change from baseline |
−2.0 |
−7.0 |
— |
|
mNAPSI change from baseline† |
−0.4 |
−2.7 |
— |
|
Nail psoriasis resolution‡ |
14.5 |
45.9 |
— |
|
HAQ-DI change from baseline |
−0.07 |
−0.38 |
<0.001 |
|
SF-36 PCS change from baseline |
1.4 |
7.3 |
<0.001 |
Safety
There were no new safety signals; however, an increase in TEAEs such as nasopharyngitis, oral candidiasis, upper respiratory infection, and fungal infections (including oral candidiasis; Table 5) was observed. Overall, there were more severe TEAEs in the bimekizumab arm compared with the placebo arm (1.9% vs 0%); although, this was not associated with an increase in study discontinuation. Drug-related TEAEs occurred in 13.1% of patients on bimekizumab versus 3% in the placebo group. No deaths were recorded.
Table 5. Safety up to Week 16*
|
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BZK, bimekizumab; Q4W, every 4 weeks; TEAE, treatment-emergent adverse events; ULN, upper limit of normal; URTI, upper respiratory tract infection. |
||
|
TEAE, % (unless otherwise stated) |
Placebo |
BZK 160 mg Q4W |
|---|---|---|
|
Any TEAE |
33.3 |
40.4 |
|
Serious TEAEs |
0 |
1.9 |
|
Discontinued due to TEAEs |
0 |
0.7 |
|
Drug-related TEAEs |
3.0 |
13.1 |
|
Severe TEAEs |
0 |
1.9 |
|
Most frequent TEAEs† |
||
|
Nasopharyngitis |
0.8 |
3.7 |
|
Oral candidiasis |
0 |
2.6 |
|
URTI |
1.5 |
2.2 |
|
Fungal infections |
0 |
4.5 |
|
Neutropenia |
0 |
1.5 |
|
Injection site reactions |
0 |
1.1 |
|
Liver function tests |
|
|
|
ALT >3 × ULN |
0 |
0.7 |
|
AST or ALT >3 × ULN |
0 |
1.5 |
Conclusion
Results of the above trials demonstrate bimekizumab as an effective treatment for PsA, with both studies meeting their primary endpoint of ACR 50. While BE COMPLETE showed an improvement in skin, joints, nails, and physical functioning in bimekizumab-treated patients who had an inadequate response or were intolerant to TNFis compared with the placebo; BE OPTIMAL established bimekizumab’s efficacy across skin and joint end points up to Week 52 in patients who were biologic DMARD-naïve. Safety profiles in both studies were consistent with previous results.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

