All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Bimekizumab for patients with plaque psoriasis
Do you know... Bimekizumab has been investigated for the treatment of patients with plaque psoriasis. In the BE VIVID, BE READY, and BE SURE trials approximately what percentage of patients in the bimekizumab treatment groups achieved a Psoriasis Area and Severity Index greater than or equal to 90% improvement from baseline (PASI90) score at Week 16?
The interleukin (IL)-17/IL-23 immunological pathway is thought to play a critical role in the development of psoriasis.1 Bimekizumab is a humanized monoclonal IgG1 antibody that inhibits IL‑17A and IL‑17F and has demonstrated positive results in multiple phase III trials. In this article, we summarize the recent approvals of bimekizumab, as well as a review by Egídio Freitas, et al.1 published in Drugs that examined its current safety and efficacy data.
Bimekizumab has received five approvals in the last year. In August 2021, bimekizumab received approval from the European Commission for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systematic therapy.2 This was quickly followed by the UK’s National Institute for Health and Care Excellence (NICE) recommending bimekizumab for patients with moderate to severe plaque psoriasis who have not responded to other treatments.3 Then, in January 2022, Japan approved bimekizumab for the treatment of plaque psoriasis, generalized pustular psoriasis, and psoriatic erythroderma in patients who are not responding sufficiently to existing treatments.4 In February 2022, Health Canada approved bimekizumab for adults with moderate to severe plaque psoriasis.5 Finally on April 7, 2022, the Australian Therapeutic Goods Administration approved bimekizumab for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy.6
However, in May 2022, the U.S. Food and Drug Administration (FDA) announced that it could not accept the Biologics License Application (BLA) for bimekizumab for the treatment of adults with moderate to severe plaque psoriasis in its current form. A complete response letter was issued stating that pre-approval inspection observations need to be resolved before the BLA can progress.7
Bimekizumab mode of action1
IL‑17 has six distinct isoforms (A−F) with IL‑17A and IL‑17F being the most similar. Bimekizumab inhibits both IL‑17A and IL‑17F by binding to an amino acid region shared by both isoforms. Both IL‑17A and IL‑17F bind to the same receptor complex (IL‑17RA/RC), as shown in Figure 1.
Figure 1. Modes of action of IL-17 inhibitors*
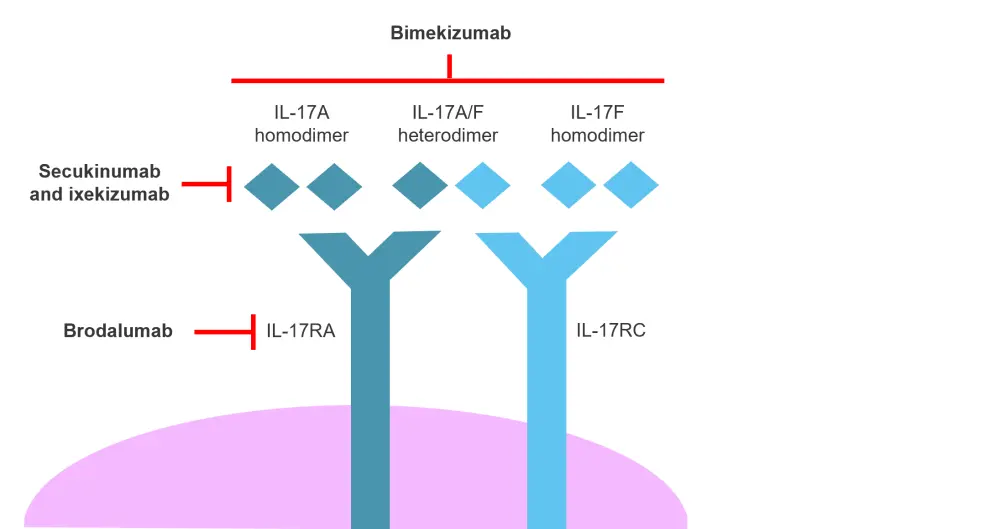
IL, interleukin.
*Adapted from Freitas, et al.1
Bimekizumab functions as a dual inhibitor of IL-17A and IL-17F, but unlike the IL-17RA inhibitor brodalumab, signaling via other isoforms, such as IL-17C and IL-17E, is unaffected. Bimekizumab can block the action of IL-17A and IL-17F homodimers as well as IL-17A/IL-17F heterodimers (Figure 1). In preclinical studies, bimekizumab has demonstrated an equal affinity for IL-17A as ixekizumab, but greater affinity compared with secukinumab. Bimekizumab is the only inhibitor in this group that can block the action of IL-17F.
By inhibiting both IL-17A and IL-17F, bimekizumab has been shown to produce a more significant decrease in inflammatory cell migration, production of pro-inflammatory cytokines, and translation of pro-inflammatory genes than inhibition of IL-17A alone.
Bimekizumab efficacy in treating patients with psoriasis1
Bimekizumab is currently being investigated for the treatment of a number of different indications, including psoriasis and psoriatic arthritis. A summary of the design and endpoints of recent phase III clinical trials for bimekizumab in patients with plaque psoriasis is shown in Table 1. Bimekizumab has been compared with ustekinumab (IL-23/IL-12 inhibitor), adalimumab (TNF-α inhibitor), secukinumab (IL-17A inhibitor), or placebo alone in the BE VIVID, BE SURE, BE RADIANT, and BE READY trials, respectively.
Table 1. Bimekizumab phase III trials designs and endpoints*
|
DB, double blinded; DLQI, Dermatology Quality of Life Index; IGA, Investigator’s Global Assessment; PASI, Psoriasis Area and Severity Index; PASI75, ≥75% improvement from baseline in PASI; PASI90, ≥90% improvement from baseline in PASI; PASI100, ≥100% improvement from baseline in PASI; q1w, every week; q4W, every 4 weeks, q8W every 8 weeks; q12w, every 12 weeks. |
||
|
Trial name |
Study design |
Primary and secondary endpoints |
|---|---|---|
|
BE VIVID |
Placebo-controlled, DB, multicenter study (N = 567) Patients treated with: |
Primary: Secondary: |
|
BE READY |
Placebo-controlled trial (N = 435) Patients treated with: |
Primary: Secondary: |
|
BE SURE |
N = 478 Patients treated with: |
Primary: Secondary: |
|
BE RADIANT |
N = 743 Patients treated with: |
Primary: Secondary: Additional secondary endpoints: |
Bimekizumab attained significantly increased Psoriasis Area and Severity Index ≥90% improvement from baseline (PASI90) scores in the BE VIVID, BE SURE, and BE READY trials at Week 16 compared with each comparator agent. The BE RADIANT trial used PASI 100% improvement from baseline (PASI100) at Week 48 as the primary study endpoint, which is shown in Figure 2. Bimekizumab significantly increased the proportion of patients achieving PASI100 compared with secukinumab. In addition, Investigator’s Global Assessment (IGA) with ≥2 categories of improvement was significantly greater for bimekizumab against all agents tested.
Figure 2. Primary endpoint results from the four trials*
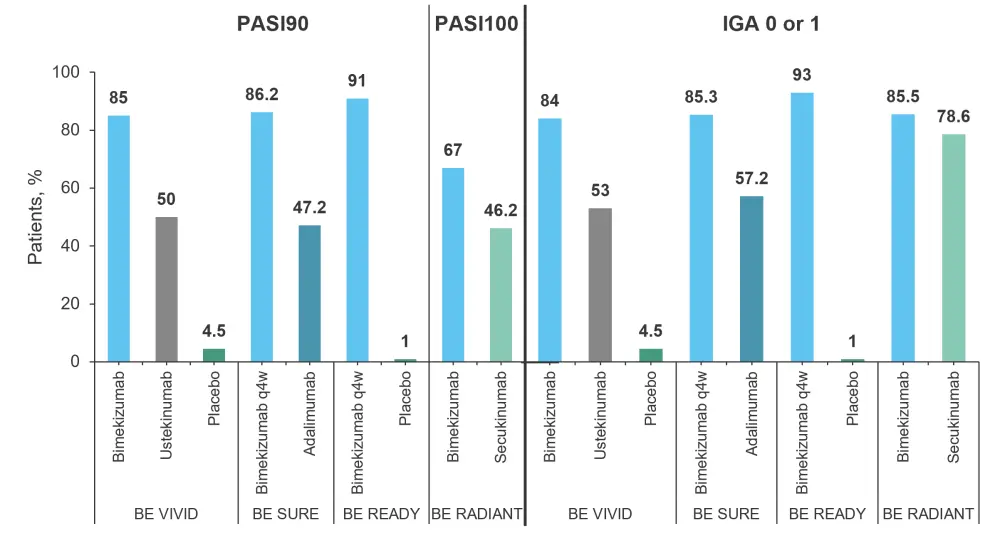
IGA, Investigator’s Global Assessment; PASI90, Psoriasis Area and Severity Index ≥90% improvement from baseline; PASI100, Psoriasis Area and Severity Index 100% improvement from baseline; q4W, every 4 weeks, q8W every 8 weeks.
*Data from Freitas, et al.1
Safety profile of bimekizumab1
Adverse event (AE) occurrence was similar between treatment groups across the four trials, with the biggest difference being seen on the BE READY trial (61% bimekizumab vs 41% placebo; Figure 3).
Figure 3. Comparison of AE incidence across the four trials*†
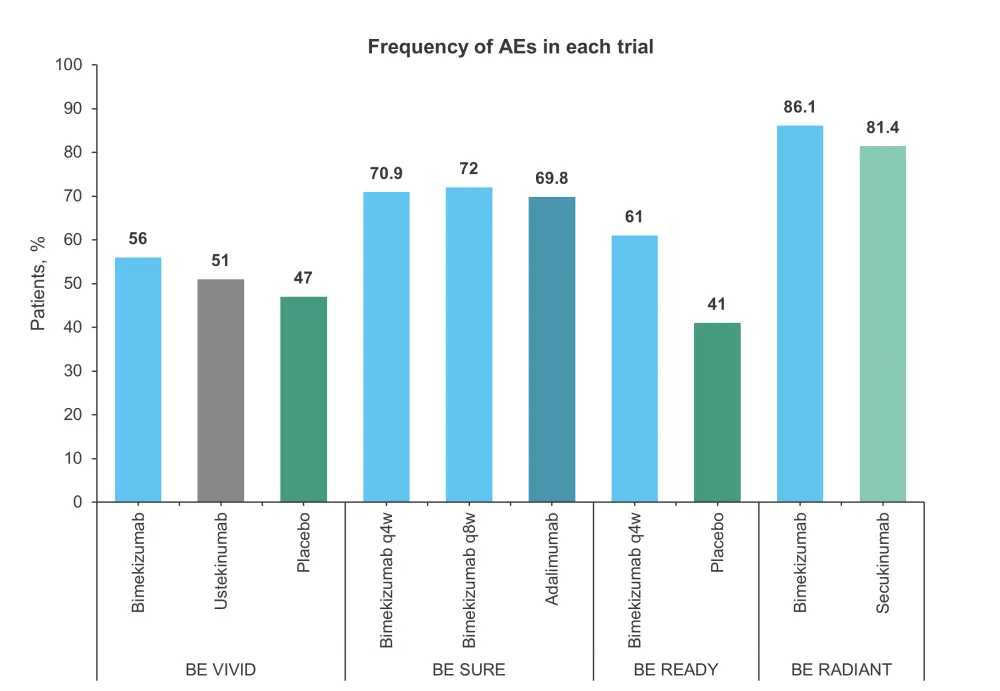
AE, adverse event; q4W, every 4 weeks, q8W every 8 weeks.
*Data from Freitas, et al.1
†Time period of assessment: 16-week initial treatment period in BE VIVID, BE READY, and BE RADIANT, and 24-week treatment period for BE SURE.
The most common AEs noted across all trials were infections such as nasopharyngitis, upper respiratory tract infections, and oral candidiasis. Most cases of oral candidiasis were mild to moderate and none were reported as being severe enough to result in treatment discontinuation in the BE READY, BE SURE, and BE RADIANT trials. In the BE VIVID trial, out of the 60 cases of oral candidiasis, three led to discontinuation of bimekizumab. Overall, oral candidiasis was slightly higher in patients treated with bimekizumab but otherwise the safety profile was similar to that of the other agents tested.
Serious AEs were recorded in three trials and the results are shown in Table 2. Overall serious AEs were low, with the BE RADIANT trial showing the highest incidence of 5.9% for bimekizumab and 5.7% for secukinumab. The BE READY trial only recorded severe AEs, which occurred in 2% of patients in both the bimekizumab and placebo arms in the first 16 weeks.
Table 2. Serious AEs at Week 16*
|
AE, adverse event; q4W, every 4 weeks, q8W every 8 weeks. |
||
|
Trial name |
Agent |
Serious AEs, %† |
|---|---|---|
|
BE VIVID |
Bimekizumab |
2 |
|
Ustekinumab |
3 |
|
|
Placebo |
2 |
|
|
BE SURE |
Bimekizumab q4w |
2.5 |
|
Bimekizumab q8w |
0.6 |
|
|
Adalimumab |
3.1 |
|
|
BE RADIANT |
Bimekizumab |
5.9 |
|
Secukinumab |
5.7 |
|
Conclusion
Bimekizumab outperformed ustekinumab, adalimumab, and secukinumab in all primary endpoints of the phase III BE SURE, BE VIVID, and BE RADIANT trials, and its safety profile was acceptable for the treatment of patients with psoriasis. Mild to moderate oral candidiasis was seen at a higher frequency with bimekizumab than with the other treatments tested, but otherwise the safety profiles were similar. Therefore, dual inhibition of IL-17A and IL-17F with bimekizumab appears to be an effective option for treating patients with psoriasis.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

