All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
The latest data on deucravacitinib and risankizumab for plaque psoriasis from WCD 2023
In recent years, several biologic treatments have been investigated for plaque psoriasis, with targets including interleukins (ILs), and tyrosine kinase 2 (TYK2). Data on two psoriasis biologics, the IL-23 inhibitor risankizumab and TYK2 inhibitor deucravacitinib, were presented at the 25th World Congress of Dermatology (WCD).1,2
Deucravacitinib
Deucravacitinib is an oral TYK2 inhibitor. The mechanism of action of deucravacitinib has been previously described on the Psoriasis and Psoriatic Arthritis Hub, along with the study designs of the POETYK PSO-1 and -2 trials. Updated results from POETYK PSO-1 and -2 up to Week 112 are now available for patients who received deucravacitinib continuously from Day 1, plus updated results from the POETYK PSO-4 trial.1
POETYK PSO-1 and PSO-2 LTE1
From the POETYK PSO-1 and PSO-2 trials, 513 patients who had received deucravacitinib continuously entered the long-term extension; of these patients, 313 had a 75% improvement in their Psoriasis Area and Severity Index (PASI 75) at Week 16.
Results from the long-term extension were:
- PASI 75 response, PASI 90 response, and static Physician’s Global Assessment score of 0 (clear) or 1 (almost clear) (sPGA 0/1) remained consistent from Week 52 to Week 112 (Figure 1A).
- PASI 75 response, PASI 90 response, and sPGA 0/1 was also maintained to Week 112 in patients who achieved PASI 75 at Week 16 (Figure 1B).
Figure 1. A Responses in all patients who received deucravacitinib continuously and B responses in patients who achieved PASI 75 at Week 16*†
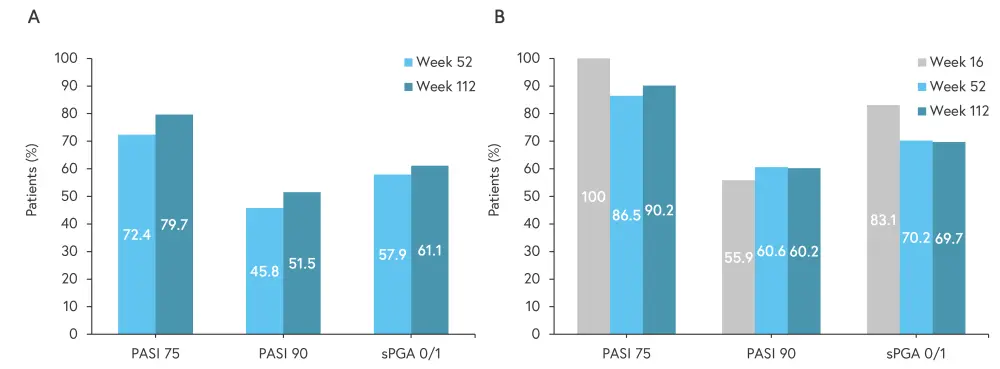
PASI 75, 75% improvement in Psoriasis Area and Severity Index; PASI 90, 90% improvement in Psoriasis Area and Severity Index; sPGA, static physician’s global assessment.
*Adapted from Lebwohl.1
†Using as observed imputation method.
POETYK PSO-42
POETYK PSO-4 was a 52-week, single-arm, multi-center, open label phase III study of adult Japanese patients with severe plaque psoriasis, generalized pustular psoriasis (GPP), or erythrodermic psoriasis (EP). Patients received deucravacitinib 6 mg once daily.
Changes were assessed using patient-reported outcomes including the Psoriasis Symptoms and Signs Diary (PSSD), which assesses 5 skin symptoms and 6 skin signs, and the Dermatology Life Quality Index (DLQI). The mean PSSD total score change from baseline (as observed) was −28.9 (95% confidence interval [CI], −33.8 to −23.9) at 16 weeks and −35.3 (95% CI, −40.9 to −29.7) at 52 weeks for patients with plaque psoriasis. At Week 16, PSSD mean score change from baseline was −24.6 (95% CI, −42.6 to −6.6) for patients with GPP and 24.4 (95% CI, -50.2–1.3) for patients with EP. At Week 52, PSSD mean score change from baseline was −19.6 (95% CI, −27.5 to −11.8) for patients with GPP and −29.4 (95% CI, −62.7–4) for patients with EP. The mean score change from baseline in individual PSSD items is shown in Figure 2.
Figure 2. Mean score change from baseline in PSSD items*
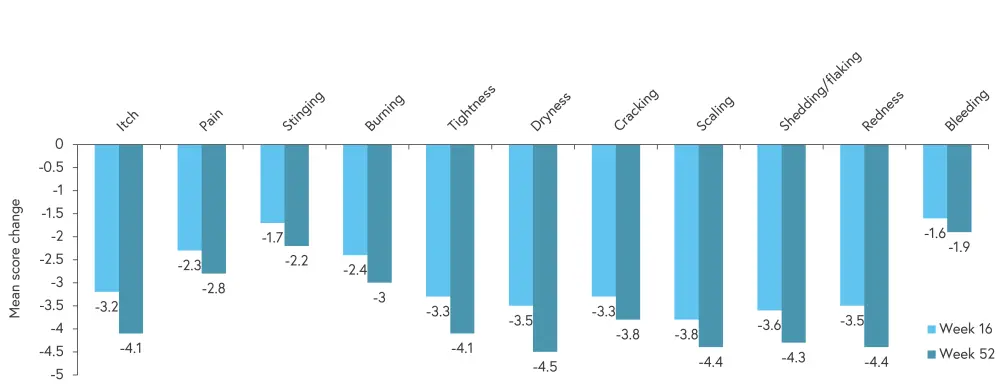
*Adapted from Tada.2
Changes were also observed in DLQI scores from baseline to Week 16 and Week 52:
- In patients with plaque psoriasis, mean change from baseline was −7.0 (95% CI, −8.1 to −6) at Week 16 and −7.6 (95% CI, −8.8 to −6.4) at Week 52.
- In patients with GPP, mean change from baseline was −8.0 (95% CI, −23.1 to 7.1) at Week 16 and −8.3 (95% CI, −27 to 10.3) at Week 52.
- In patients with EP, mean change from baseline was −6.6 (95% CI, −11.7 to −1.4) at Week 16 and −7.8 (95% CI, −13.8 to 1.9) at Week 52.
Risankizumab
Risankizumab is a monoclonal antibody used treat moderate-to-severe plaque psoriasis by blocking the action of IL23. The efficacy of risankizumab for the treatment of psoriatic arthritis has previously been reported on the Psoriasis and Psoriatic Arthritis Hub.
KNOCKOUT3
KNOCKOUT is an ongoing, single center phase II study in adult patients with plaque psoriasis. Patients were randomized 1:1 to risankizumab 600 mg or 300 mg for 16 weeks; follow-up was up to 100 weeks. As of June 13, 2023, 17 patients had reached Week 40.
In pooled data from both dosing groups, mean PASI score had improved from 19.1 at baseline to 0.6 at Week 40, with a mean improvement of 97%. PASI responses at Week 40 are shown in Figure 3. The safety profile in this study was similar to previous studies of risankizumab.
Figure 3. PASI responses at Week 16 and 40*
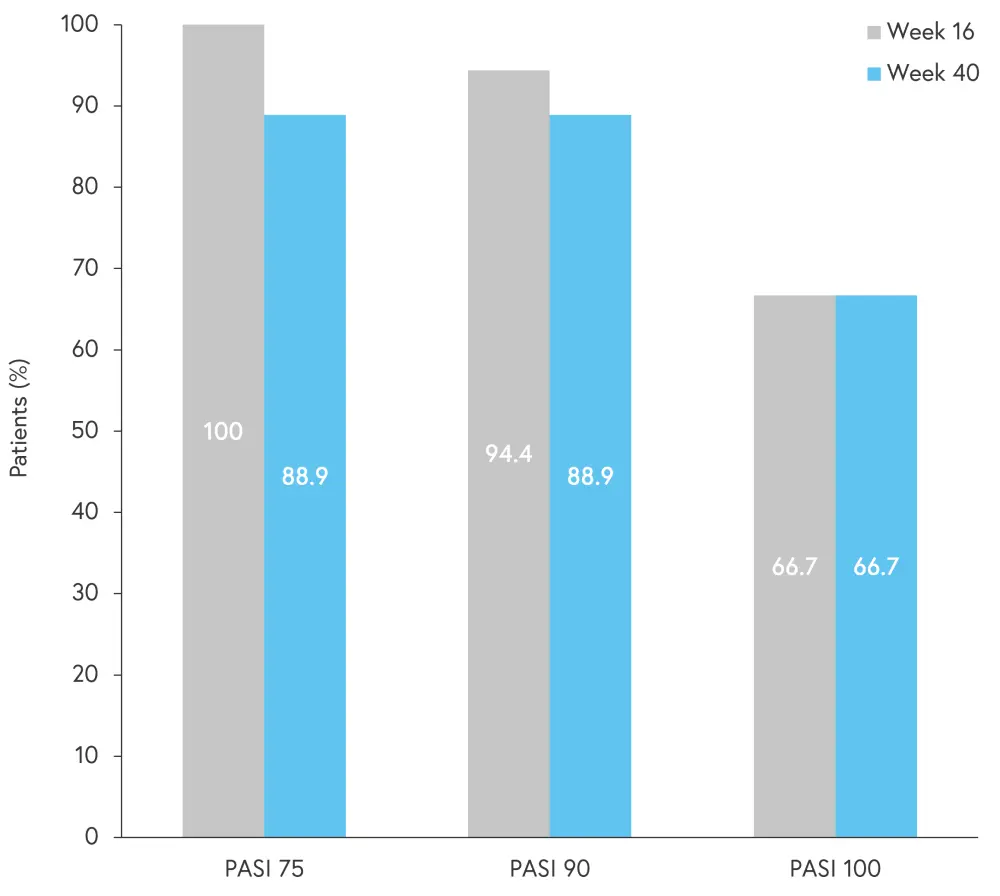
PASI 75, 75% improvement in Psoriasis Area and Severity Index; PASI 90, 90% improvement in PASI; PASI 100, 100% improvement in PASI.
*Data from Blauvelt.3
Conclusion
Patients treated with deucravacitinib in the POETYK PSO-1, POETYK-PSO-2, and POETYK-PSO-4 trials experienced long-term efficacy, as measured by PASI score, sPGA, and patient reported outcomes.1,2 These studies suggest that once daily treatment with deucravacitinib is effective for use long-term in patients with plaque psoriasis, and can improve patient quality of life.2
Similarly, treatment with risankizumab demonstrated efficacy up to Week 40 as measured by PASI 75 and PASI 90 responses, with no new safety signals.3 These results demonstrate that deucravacitnib and risankizumab can be used long-term in patients with plaque psoriasis.1,2,3 Results from POETYK PSO-4 also suggest that deucravacitinib treatment can improve quality of life in patients with EP and GPP, although these results are limited by a small patient population.2
Your opinion matters
Which criteria or attribute is most important to you when selecting a biological therapy for a patient with moderate to severe psoriasis?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

