All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Long-term efficacy of deucravacitinib and COVID-19-related adverse events in patients with plaque psoriasis
Tyrosine kinase 2 (TYK2) is an intracellular kinase that is a member of the Janus kinase (JAK) family. TYK2 is responsible for facilitating the signaling of cytokines, such as interleukin (IL)-23, that are involved in the pathogenesis of psoriasis, psoriatic arthritis, and other immune-mediated diseases.1,2
Deucravacitinib is a novel, oral, selective TYK2 inhibitor, recently approved by the U. S. Food and Drug Administration for the treatment of adults with moderate-to-severe plaque psoriasis. Deucravacitinib exhibits a unique mechanism of action, distinct from other JAK inhibitors, by binding to the regulatory domain of TYK2 and inhibiting the enzyme by locking it in an inactive state (Figure 1).1,2
Figure 1. Mechanism of action of deucravacitinib*
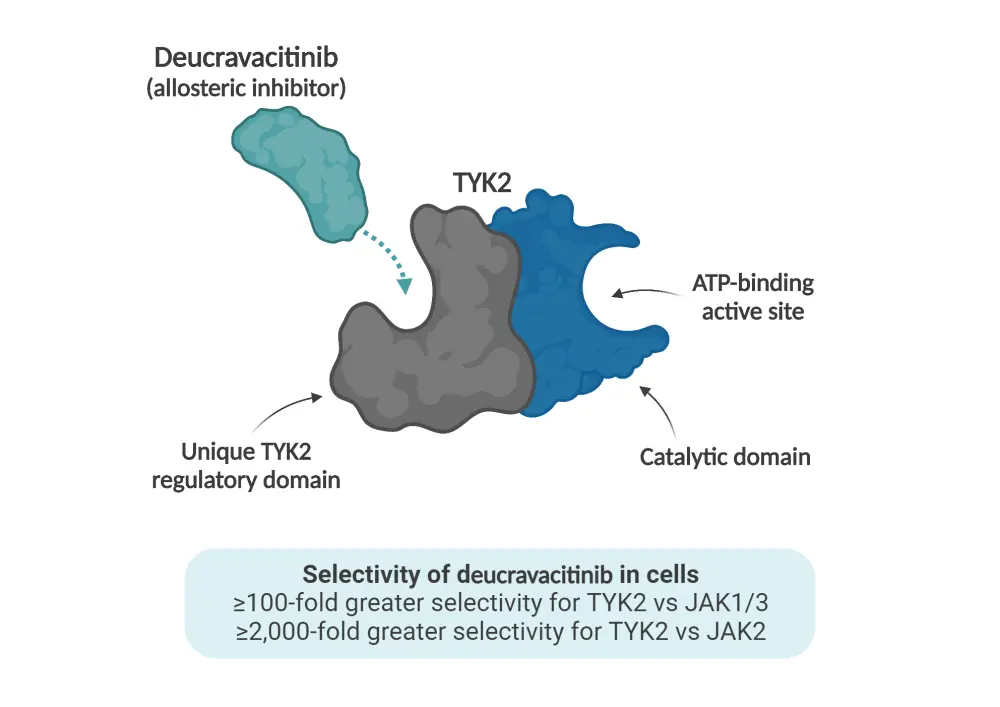
ATP, adenosine triphosphate; JAK, Janus kinase; TYK2, tyrosine kinase 2.
*Adapted from Lebwohl1 and Thaçi.2 Created with BioRender.com.
During the 31st European Academy of Dermatology and Venereology (EADV) Congress, Mark Lebwohl presented the long-term efficacy results of deucravacitinib from the POETYK LTE trial (NCT04036435),1 and Diamant Thaçi presented on COVID-19-related adverse events (AEs) in patients with plaque psoriasis treated with deucravacitinib in three trials; POETYK-PSO-1 (NCT03624127), POETYK-PSO-2 (NCT03611751), and POETYK LTE.2 We are pleased to provide a summary of these presentations here.
Study designs
POETYK-PSO-1 and POETYK-PSO-2 were multicenter, randomized, phase III trials in patients aged ≥18 years, diagnosed with moderate-to-severe plaque psoriasis with a Psoriasis Area and Severity Index (PASI) score of ≥12, static Physician’s Global Assessment (sPGA) score of ≥3, and body surface area involvement of ≥10%.1,2
POETYK-PSO-1 was a 52-week crossover trial in which patients were randomized to deucravacitinib, placebo, or apremilast (Figure 2). This trial demonstrated achievement and sustained clinical efficacy through Week 52. Patients randomized to deucravacitinib on Day 1 in POETYK-PSO-1 and who continued the treatment beyond 52 weeks were eligible to enter the POETYK LTE (long-term extension) open-label trial (Figure 2).1
For the efficacy analysis discussed by Lebwohl, the populations were defined as:
- patients who received continuous deucravacitinib treatment from baseline and entered the LTE trial; or
- patients who received continuous deucravacitinib from baseline, achieved PASI 75 at Week 16, and entered the LTE trial.1
In this efficacy analysis, the outcome measures of POETYK LTE were PASI 75, PASI 90, and sPGA 0 or 1 at ≤112 weeks of continuous deucravacitinib treatment.1
The following two methods of imputation for missing data were used to evaluate long-term efficacy1:
- Treatment failure rules; patients who discontinued treatment or the study due to worsening of psoriasis or lack of efficacy were imputed as non-responders.
- Modified non-responder imputation; multiple imputation analysis was used for imputation of missing values and patients who discontinued treatment due to worsening psoriasis were imputed as non-responders.
As discussed by Thaçi,2 the analysis of COVID-19-related AEs compared deucravacitinib-treated patients from the POETYK PSO-1, POETYK PSO-2, and POETYK LTE trials with a placebo group of the COVID-19 vaccine trial (NCT04505722).2 The outcomes included the incidence rates, severity, distribution by country and age group of COVID-19-related AEs, including any leading to treatment discontinuation, as well as a summary of COVID-19-related AEs according to COVID-19 vaccination status.2
Figure 2. Study designs of the POETYK trials*
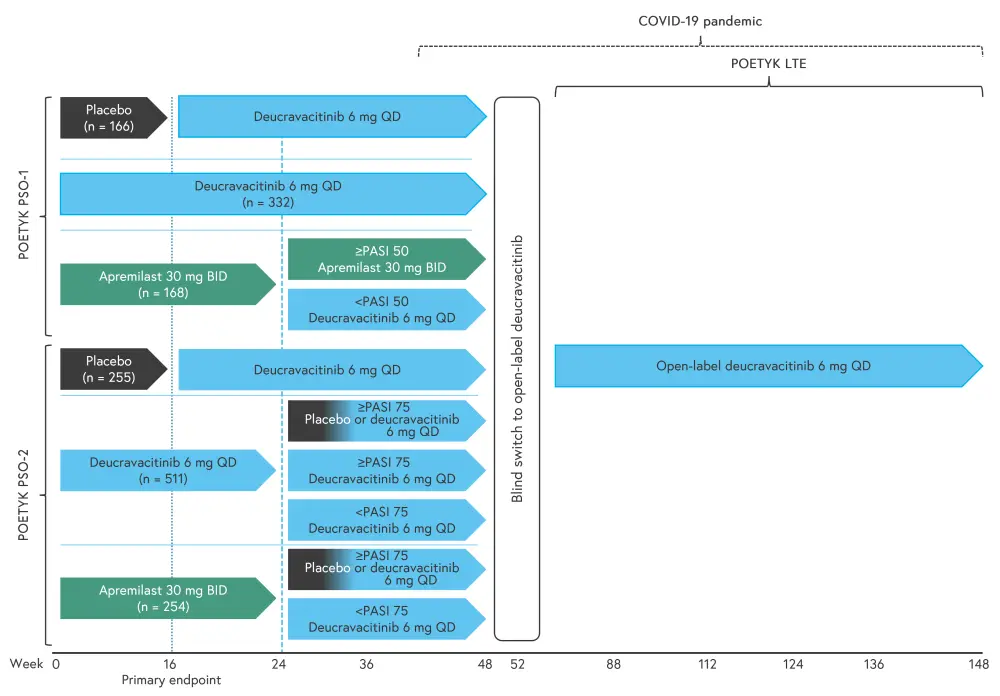
BID, twice daily; PASI, Psoriasis Area Severity Index; QD, once daily.
*Adapted from Lebwohl1 and Thaçi.2
Results
Both presentations used a data cut-off date of October 1, 2021.1,2 For the long-term efficacy analysis presented by Lebwohl, this was at Week 112.1
Baseline characteristics
A total of 265 patients entered POETYK LTE, including 173 patients who achieved PASI 75 at Week 16.1 The mean age was 46 years, mean age at disease onset was 29.8 years, and 32.8% of patients were female.1
A total of 1,364 patients met the criteria for analysis of COVID-19-related AEs, with 2,076.7 person-years (PY) of follow-up.2 The mean age was 46.6 years and 32.2% of patients were female.2 Selected baseline characteristics from POETYK LTE and the study of COVID-19-related AEs are shown in Table 1.
Table 1. Baseline characteristics of patients*
|
AE, adverse event; BSA, body surface area; LTE, long-term extension; PASI, Psoriasis Area and Severity Index; SD, standard deviation; sPGA, static Physician’s Global Assessment. |
|||
|
Characteristic, % (unless stated otherwise) |
Efficacy assessment of patients in the POETYK LTE trial |
Assessment of COVID‑19-related AEs |
|
|---|---|---|---|
|
Total (n = 265) |
Week 16 PASI 75 responders (n = 173) |
Population at risk for COVID-19 (n = 1,364) |
|
|
Mean weight, kg (SD) |
87.0 (22.2) |
84.7 (22.4) |
90.1 (21.3) |
|
Ethnicity |
|
||
|
White |
79.6 |
76.9 |
87.2 |
|
Asian |
19.2 |
21.4 |
10.3 |
|
Black or African American |
0.4 |
0.6 |
1.5 |
|
Mean PASI (SD) |
21.8 (8.25) |
22.6 (8.9) |
21 (8.1) |
|
sPGA |
|
||
|
3 (moderate) |
78.5 |
74.6 |
80.0 |
|
4 (severe) |
21.5 |
25.4 |
20.0 |
|
Mean BSA involvement, % (SD) |
27.2 (15.6) |
28.3 (15.6) |
26.2 (15.7) |
PASI 75, PASI 90, and sPGA1
PASI 75 response rate was consistent, with a response rate of 84.4% (as observed) at Week 112, and non-responder imputations did not markedly change response rate (Figure 3). Clinical efficacy with continuous deucravacitinib was maintained in all patients at 112 weeks.
Figure 3. PASI 75, PASI 90, and sPGA response rates at A Week 52 and B Week 112*
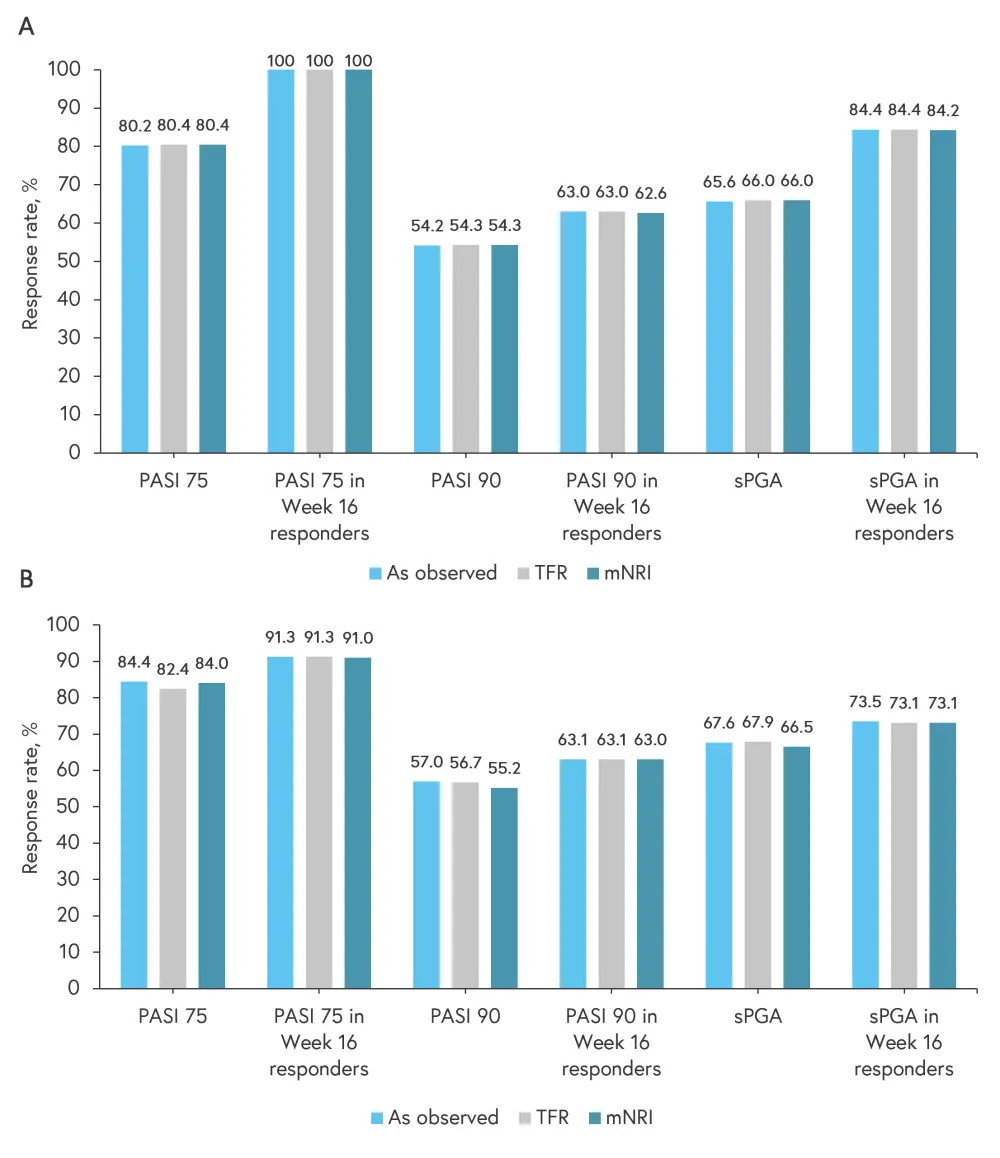
mNRI, modified non-responder imputation; PASI, Psoriasis Area and Severity Index; sPGA, static Physician’s Global Assessment; TFR, treatment failure rules.
*Data from Lebwohl.1
Incidence rates and severity of COVID-19-related AEs2
COVID-19-related AEs, serious COVID-19-related AEs (SAEs), and deaths were seen in 153, 43, and 6 patients, with exposure-adjusted incidence rates (EAIRs) of 7.6 per 100 PY (95% confidence interval [CI], 6.5–8.9), 2.1 per 100 PY (95% CI, 1.5–2.8), and 0.3 per 100 PY (95% CI, 0.1–0.6), respectively. The EAIRs for COVID-19-related SAEs and deaths in the reference population (placebo) were 16.5 per 100 PY (95% CI, 15.0–17.9) and 0.23 per 100 PY (95% CI, 0.1–0.5), respectively. There were no differences observed in the incidence rates and severity of COVID-19-related AEs in patients across the different countries.
With regards to age distribution of patients with COVID-19-related AEs and SAEs, most patients were in the 50–64 years age group (Figure 4). Five of the patients who died were in the 50–64 years age group and one was in the 75–84 years age group.
Figure 4. Age distribution of patients experiencing COVID-19-related AEs and SAEs*
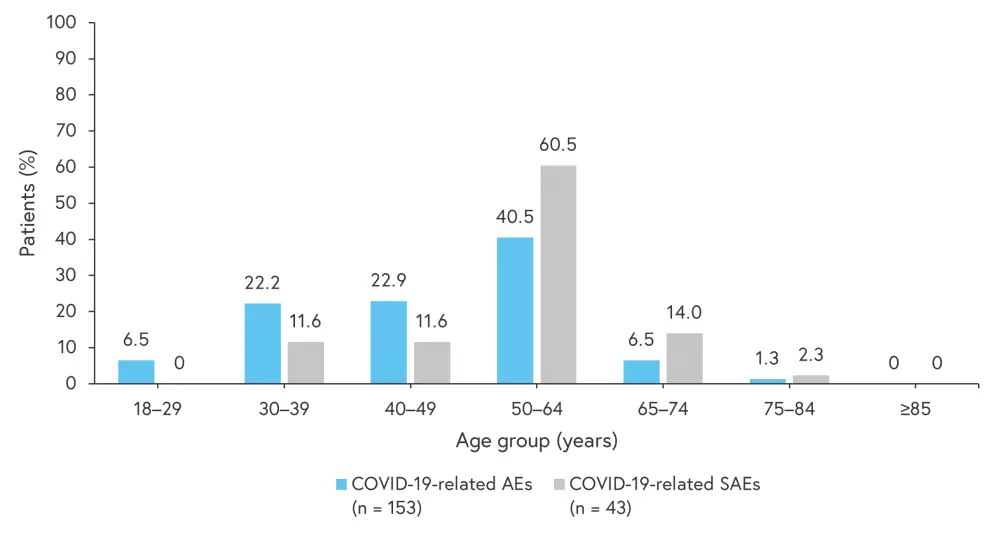
AE, adverse events; SAE, serious adverse event.
*Adapted from Thaçi.2
A total of seven patients discontinued treatment due to COVID-19-related AEs, six of which were serious (Table 2).
Table 2. COVID-19-related AEs and SAEs leading to treatment discontinuation*
|
AEs, adverse event; EAIR, exposure-adjusted incidence rate; PY, person-years; SAEs, serious adverse events. |
|||
|
AEs and SAEs |
n |
PY |
EAIR/100 PY |
|---|---|---|---|
|
AEs |
|
|
|
|
Total patients with an event |
7 |
2,077.6 |
0.34 |
|
COVID-19-related |
6 |
2,077.3 |
0.29 |
|
COVID-19 pneumonia |
1 |
2,077.0 |
0.05 |
|
SAEs |
|
|
|
|
Total patients with an event |
6 |
2,077.6 |
0.29 |
|
COVID-19-related |
5 |
2,077.3 |
0.24 |
|
COVID-19 pneumonia |
1 |
2,077.0 |
0.05 |
COVID-19-related AEs in the POETYK LTE trial by vaccination status2
A total of 1,219 patients were included in this analysis. No vaccines were reported in 107, 33, and 5 patients with COVID-19-related AEs, SAEs, and death, respectively (Table 3).
Table 3. COVID-19-related AEs by vaccination status in POETYK LTE trial*
|
AE, adverse event; NA, not applicable; SAE, serious adverse event. |
|||
|
Vaccination status, % |
COVID-19-related AE |
COVID-19-related SAE |
COVID-19-related death |
|---|---|---|---|
|
≥1 dose of COVID-19 vaccine prior to event |
4.8 |
2.4 |
16.7 |
|
No vaccine reported |
73.8 |
80.5 |
83.3 |
|
First dose of COVID-19 vaccine after event |
21.4 |
17.1 |
NA |
Conclusion
The POETYK LTE trial demonstrated the durable efficacy of deucravacitinib for up to 112 weeks in patients with plaque psoriasis. The outcomes were consistent in this population from Week 52 to Week 112 and were maintained among those patients who achieved PASI 75 by Week 16. Lebwohl concluded that deucravacitinib, a once daily oral medication, has the potential to become the treatment of choice, with an efficacy similar to that seen with biologic agents.
Although, COVID-19-related AEs were among the most frequently reported AEs during the 2-year follow-up period, these were not serious and did not lead to treatment discontinuation. Compared with the reference population from the placebo arm of the COVID-19 vaccine trial, the incidence rates and severity of COVID-19-related AEs were similar to the expected infection rates. The occurrence of most AEs and SAEs in unvaccinated patients suggests that vaccination provides protection against infection in patients receiving deucravacitinib, similar to the general population. Overall, the COVID-19-related AEs study shows that deucravacitinib is not associated with an increased risk of COVID-19 infection, nor was it associated with severe COVID-19 outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

