All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Dual inhibition of IL-17A and IL-17F in psoriatic disease
Do you know... Which of the following is the only IL-17A/IL-17F dual inhibitor approved by the FDA and EC for the treatment of adult patients with plaque psoriasis and psoriatic arthritis?
During the PsOPsA Hub Steering Committee Meeting on July 16, 2025, Peter Nash, Griffith University, Queensland, AU, chaired a discussion on dual inhibition of IL-17A and IL-17F in psoriatic disease. The discussion also featured Paolo Gisondi, Ulrich Mrowietz, Yukari Okubo, and Jody Quinn.
Dual inhibition of IL-17A and IL-17F in psoriatic disease
Dual inhibition of IL-17A and IL-17F in psoriatic disease
Presentation
Nash presented an overview of dual interleukin (IL)-17A and IL-17F inhibitors – bimekizumab, sonelokimab, and ORKA-002 – highlighting their mechanism of action and summarizing key clinical evidence in psoriatic disease.
- Differential regulation of IL-17A and IL-17F via signal transducer and activator of transcription 5 (STAT5) contributes to psoriatic disease, and dual or bispecific inhibition of IL-17A and IL-17F may provide greater efficacy than targeting IL-17A alone (Figure 1).1
- Currently, bimekizumab is the only IL-17A and IL-17F dual inhibitor approved by the U.S. Food and Drug Administration (FDA) and European Commission (EC) for the treatment of adult patients with plaque psoriasis (PsO) and psoriatic arthritis (PsA).2,3 Sonelokimab and ORKA-002 are also in development (Figure 2).
Figure 1. Role of IL-17A and IL-17F in the pathogenesis of psoriatic disease*
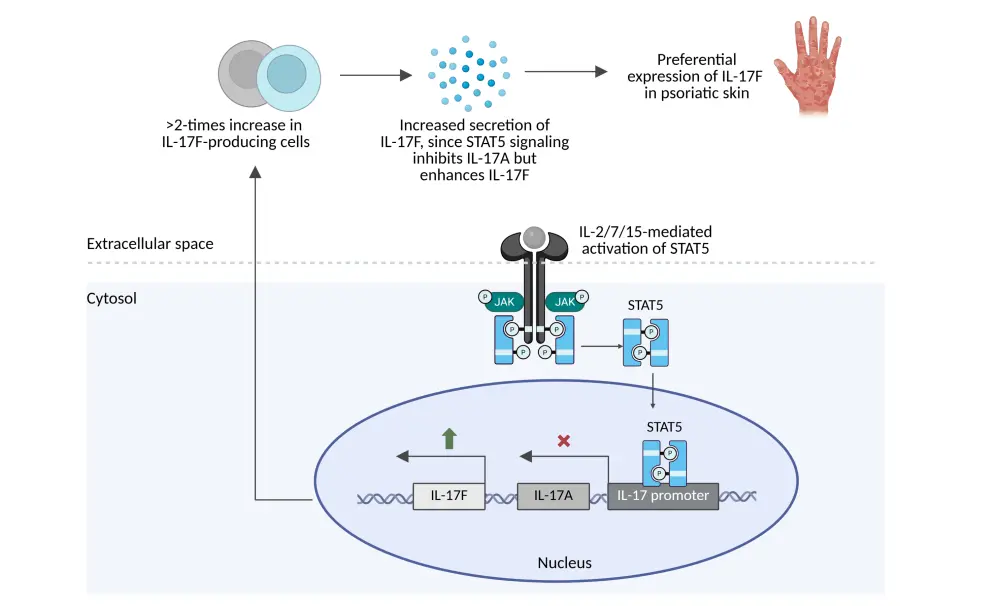
Figure 2. IL-17A and IL-17F dual inhibitors*
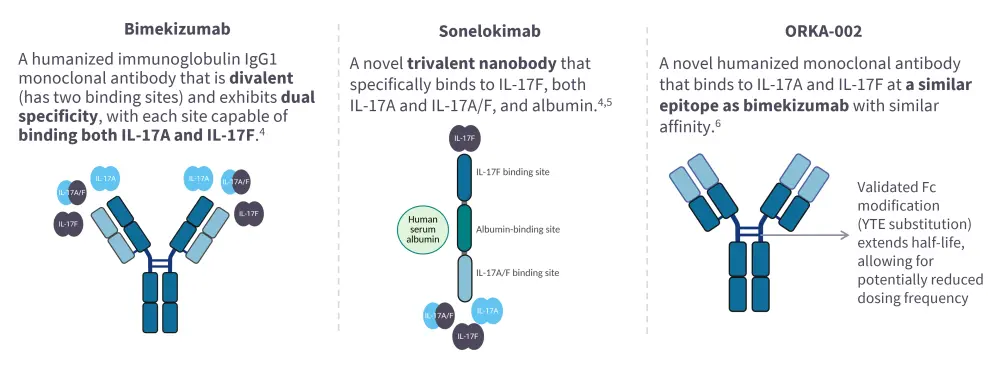
Bimekizumab
- Bimekizumab demonstrated rapid and complete skin clearance in patients with moderate-to-severe PsO in the phase II BE ABLE 1 (NCT02905006) and BE ABLE 2 (NCT03010527) trials.7, 8
- Bimekizumab showed superior efficacy compared with ustekinumab (IL-23/IL-12 inhibitor), adalimumab (tumor necrosis factor [TNF]-α inhibitor), secukinumab (IL-17A inhibitor), or placebo alone in the phase III BE VIVID (NCT03370133), BE SURE (NCT03412747), BE RADIANT (NCT03536884), and BE READY (NCT03410992) trials, respectively.9
- These findings supported the FDA approval of bimekizumab for the treatment of patients with moderate-to-severe PsO.10
- In patients with PsA, bimekizumab demonstrated efficacy across multiple disease domains, including joints, skin and nail psoriasis, dactylitis, radiographic progression, and uveitis, in those naïve to biologic DMARDs (BE OPTIMAL and open-label extension [OLE] BE VITAL) and in those with inadequate response to TNF inhibitors (BE COMPLETE and OLE BE VITAL).11–18
- Based on the findings, bimekizumab was granted FDA approval for the treatment of patients with active PsA.2
- A post-approval head-to head trial (BE BOLD; NCT06624228) of bimekizumab vs risankizumab in patients with PsA is currently ongoing.19
Sonelokimab
- Sonelokimab has demonstrated rapid and robust clinical responses compared with placebo in patients with PsO (phase IIa trial; NCT03384745)5 and PsA (phase II ARGO trial; NCT05640245).20
- Two phase III trials, IZAR-1 (NCT06641076) and IZAR-2 (NCT06641089), are currently underway to evaluate sonelokimab in patients with PsA.
ORKA-002
- ORKA-002 demonstrated around 3-fold longer half-life than bimekizumab, suggesting the potential for only 2–3 doses per year in preclinical studies.6
- A phase I first-in-human trial (NCT06944379) of ORKA-002 is currently underway.6
Discussion
- Treatment decisions in psoriatic disease are guided by both administrative and clinical factors.
- Administratively, biosimilars are recommended as first choice due to cost constraints. If treatment fails, other classes such as IL-23 (p19) or IL-17 inhibitors may be considered.
- Clinically, treatment decisions are mainly driven by disease severity. Dual IL-17A/F inhibitors are favored for patients with severe, resistant, or diffuse psoriasis, as well as in those presenting with arthralgia – even in the absence of a confirmed PsA diagnosis – compared with adalimumab and other agents.
- In Germany, all biologics are accessible, including bimekizumab.
- IL-17 inhibitors, particularly bimekizumab and ixekizumab, are the first choice for PsA.
- Bimekizumab is also preferred after failure of other biologics or other treatments, and in severe skin disease.
- Candida infections, especially folliculitis, remain a concern, particularly during the first year of treatment. Management is further complicated by the need to avoid azole antifungals, such as fluconazole, due to CYP450-mediated drug–drug interactions.
- Despite this, bimekizumab remains a highly effective option for skin, joint, and nail involvement.
- In Japan, IL-17A or IL-23 inhibitors are typically used as first-line therapy for severe skin disease, while dual IL-17A/F inhibitors are reserved for use after treatment failure. For difficult-to-manage PsA, dual inhibitors are often preferred as first-line therapy.
- Real-world incidence of Candida appears to be reduced with consistent oral hygiene.
- Patient experience of biologics found bimekizumab to give relief from psoriatic pain, and scalp and hand psoriasis, but also suggested that treatments spaced beyond 4 weeks may be to be less effective.
This independent educational activity is supported by UCB. All content was developed independently. The funder was allowed no influence on the content of this activity.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?


 Ulrich Mrowietz
Ulrich Mrowietz Jody Quinn
Jody Quinn Paolo Gisondi
Paolo Gisondi Peter Nash
Peter Nash Yukari Okubo
Yukari Okubo
