All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Selective TYK2 inhibition for the treatment of psoriatic disease
Do you know... Which of the following is an allosteric TYK2 inhibitor currently in development for the treatment of psoriatic disease?
During the PsOPsA Hub Steering Committee Meeting on July 16, 2025, Paolo Gisondi, University Hospital of Verona, Verona, IT, chaired a discussion on selective TYK2 inhibition for the treatment of psoriatic disease. The discussion also featured Ulrich Mrowietz, Peter Nash, and Yukari Okubo.
Selective TYK2 inhibition for the treatment of psoriatic disease
Selective TYK2 inhibition for the treatment of psoriatic disease
Presentation
Gisondi provided an overview of TYK2 inhibitors, describing their mechanism of action and distinguishing between allosteric and orthosteric types, with a particular focus on the allosteric inhibitors deucravacitinib and zasocitinib.1
Deucravacitinib
Deucravacitinib is approved for the treatment of plaque psoriasis (PsO) based on efficacy demonstrated in the POETYK-PSO-1 (NCT03624127) and POETYK-PSO-2 (NCT03611751) trials. Two-year follow-up results from the ongoing phase IIIb, open-label POETYK PSO-LTE (NCT04036435) trial indicated that treatment with deucravacitinib maintained or further improved efficacy through Week 112 compared with the start of the extension trial (Week 52) in adults with moderate-to-severe PsO.2
Deucravacitinib has also yielded positive results in the phase III POETYK PsA-1 (NCT04908202) and POETYK PsA-2 (NCT04908189) trials, giving higher Week 16 ACR20 response rates compared with placebo.3,4
Zasocitinib
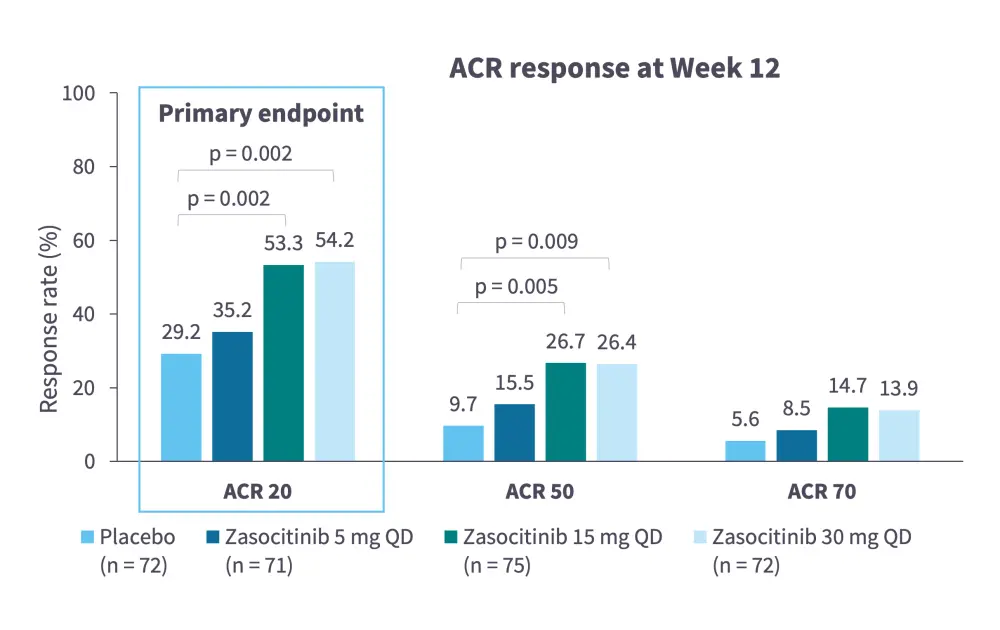
Zasocitinib is a TYK2 inhibitor in late-stage clinical development (Table 1)5–13 and has been found to be efficacious and tolerable in both PsO14 and PsA15 (Figure 1 and Figure 2).
Table 1. Zasocitinib clinical trial landscape*
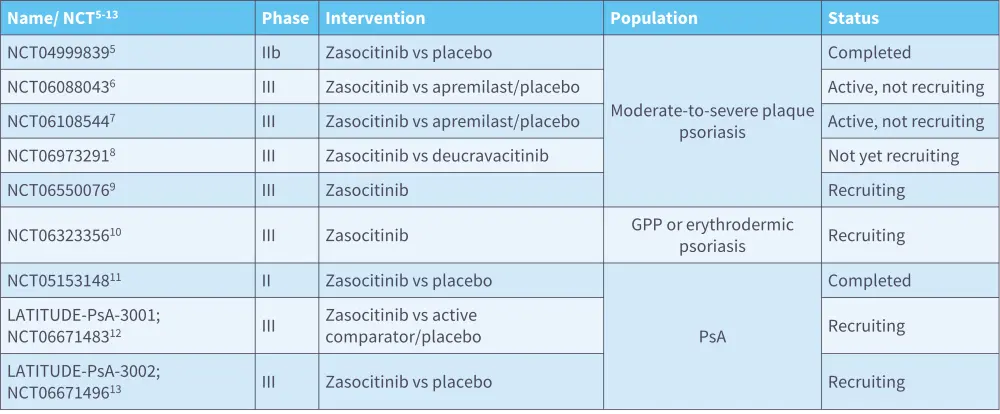
Figure 1. Zasocitinib in PsO: Efficacy results from a randomized, multicenter, double-blind, placebo-controlled, phase IIb trial (NCT04999839)*
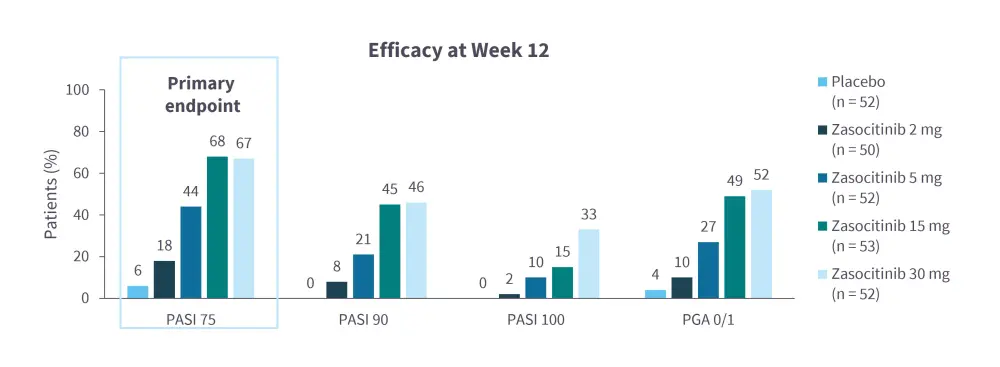
Figure 2. Zasocitinib in PsA: Efficacy results from a randomized, multicenter, double-blind, phase IIb trial (NCT05153148)*
Discussion
- The clinical utility of TYK2 inhibitors is often questioned, given their moderate efficacy (PASI 90 response rates <50%), and relatively higher incidence of AEs (e.g. folliculitis, nasopharyngitis) and infections, especially when compared with IL-23 p19 inhibitors.
- Oral administration of deucravacitinib and zasocitinib offers practical advantages, though patient preferences vary by region. Oral agents are favored in Japan and Australia for convenience, easier storage, or for patients recovering from AEs associated with biologics (especially with tumor necrosis factor inhibitors), while in Germany, injectables are preferred for their greater efficacy, less frequent dosing, and lower psychological burden.
- In Australia, oral agents remain integral to PsA management, particularly as bridging therapies before biologics. Deucravacitinib is generally well-tolerated in patients with mild-to-moderate PsA and is often used off-label in combination with traditional biologics for treatment-refractory cases.
- In Japan, apremilast is typically prescribed first due to its lower cost, with TYK2 inhibitors considered upon failure. For moderate-to-severe PsO, earlier initiation of TYK2 inhibitors is being explored, while biologics remain the preferred option in PsA.
- Real-world data from Germany indicate the highest drug survival with IL-23 p19 inhibitors, followed by IL-17 inhibitors and adalimumab. In contrast, oral treatments including apremilast and deucravacitinib show lower drug survival, due to factors such as limited efficacy and AEs, meaning they are less favorable from both clinical and economic perspectives.
This independent educational activity is supported by Takeda. All content was developed independently. The funder was allowed no influence on the content of this activity.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?


 Ulrich Mrowietz
Ulrich Mrowietz Paolo Gisondi
Paolo Gisondi Peter Nash
Peter Nash Yukari Okubo
Yukari Okubo

