All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Targeted therapy for psoriasis: A focus on tyrosine kinase 2 inhibitors
Do you know... Which of the following agents in development for the treatment of psoriasis is correctly paired with its mechanism of action?
Psoriasis is a chronic, systemic, immune-mediated, inflammatory condition, affecting 2–3% of the global population, that can substantially impact patients’ quality of life (QoL).1,2 Current treatment options include topical medications, ultraviolet-based treatments, biologics, and oral small molecules.2 While biologics have improved outcomes for patients with psoriasis, they require intravenous or subcutaneous administration and may lose efficacy over time; therefore, oral small molecule inhibitors have the potential to improve patient adherence and QoL.1 Conventional non-targeted therapies are associated with adverse events (AEs) and long-term safety issues,1 meaning there is a clinical need for safe and efficacious novel oral targeted therapies for patients with psoriasis.1,2 Tyrosine kinase 2 (TYK2) inhibitors have recently been explored as a promising therapeutic approach in this patient population.3
Below, we provide a deep dive into TYK2 inhibitors in the treatment of patients with psoriasis, their efficacy and safety, as well as how they might impact clinical practice.
Key therapeutics
Currently, there are four oral TYK2 inhibitors approved or in late-stage clinical development: deucravacitinib, zasocitinib, and brepocitinib for psoriasis and psoriatic arthritis, and ropsacitinib for psoriasis.1,2,4 Additionally, several other TYK2 inhibitors are currently being investigated in clinical trials (Table 1).
Table 1. Oral TYK2 inhibitors in clinical development*
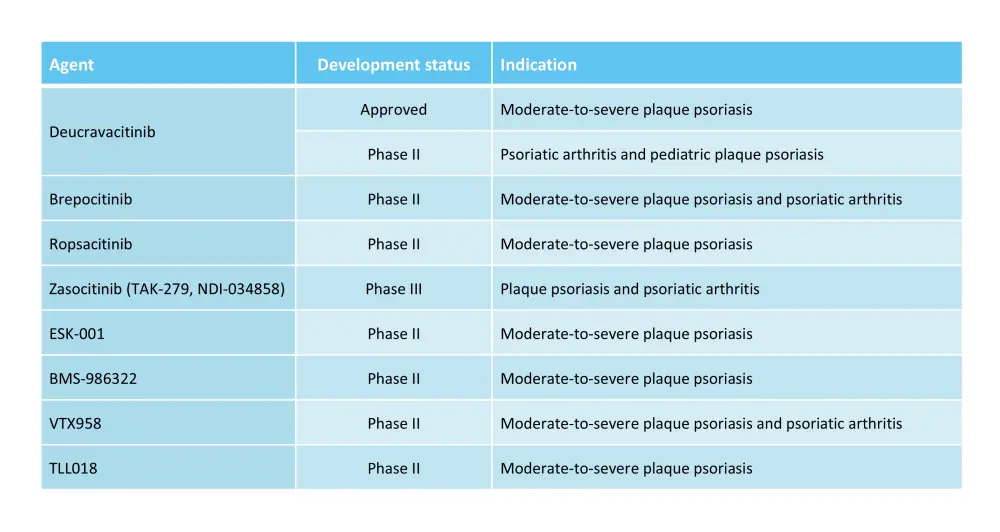
*Adapted from Hawkes, et al.2; and Dragotto, et al.4
Deucravacitinib is a first-in-class, oral, once-daily, selective, allosteric TYK2 inhibitor, approved by the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy.1,4 Deucravacitinib is also approved by the Pharmaceutical and Medical Devices Agency (PDMA) in Japan for the treatment of patients with plaque psoriasis, generalized pustular psoriasis, and erythrodermic psoriasis with an inadequate response to conventional therapy, and by the European Commission for the treatment of moderate-to-severe psoriasis.1,5
Brepocitinib is an oral, orthosteric inhibitor of TYK2 and Janus kinase (JAK) 1/2 in development for the treatment of several disorders, including psoriatic disorders.4 Ropsacitinib is an orthosteric inhibitor of TYK2 and JAK2 that is being investigated for the treatment of patients with moderate-to-severe psoriasis.4 Zasocitinib is an oral allosteric inhibitor of TYK2 currently being evaluated for the treatment of multiple autoimmune diseases, including psoriasis.2
Question 1 / 1
Which of the following TYK2 inhibitors is approved by the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy?
A
Brepocitinib
B
Zasocitinib
C
Deucravacitinib
D
Ropsacitinib
Mechanism of action
Pathogenesis of psoriasis
Immune system dysregulation, characterized by a complex interaction between genetic and immunological factors, is central to the pathogenesis of psoriasis.4 Psoriasis is characterized by persistent inflammation, resulting in excessive proliferation and abnormal differentiation of keratinocytes.4 Both the innate and adaptive immune systems contribute to the inflammatory process through a complex feedback loop mechanism.4
Advancements in our understanding of the role of the interleukin (IL)-23/IL-17 axis as the primary pathogenic pathway for psoriasis have identified potential therapeutic targets.4 IL-23 promotes inflammation by controlling the expansion and survival of T helper (Th)17 and Th22 cells, which release proinflammatory cytokines, including IL-17 and IL-22, while IL-12 stimulates Th1 cell development, which release cytokines such as tumor necrosis factor (TNF)-α and interferon (IFN)-γ.1 Both IL-23 and IL-12 signal through the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway.4
JAK/STAT signaling pathway
There are four kinases in the JAK family: JAK1, JAK2, JAK3, and TYK2.1 JAK enzymes consist of two domains: the catalytic domain and the regulatory pseudokinase domain.1 When cytokines bind to the surface receptor of the JAK enzymes, a conformational change allows the binding of adenosine triphosphate (ATP) to the catalytic domain, activating the JAK enzymes.1 The JAK/STAT pathway mediates signaling downstream of type I and II cytokines, including proinflammatory cytokines, highlighting the potential of JAK enzymes as a therapeutic target; however, the inhibition of JAK1, JAK2, and JAK3 may also disrupt important cellular functions.1 TYK2 is integral to the pathogenesis of psoriasis through the mediation of downstream signaling of IL-12, IL-23, and type I IFN-α and IFN-β receptors, and, as such, several TYK2 inhibitors are currently being evaluated.1
TYK2 inhibition
Inhibition of TYK2 signaling is possible by disrupting the connection between IL-23 and IL-17 (Figure 1).1 There are two categories of TYK2 inhibitors, allosteric inhibitors and orthosteric inhibitors.1 Allosteric inhibitors, such as deucravacitinib and zasocitinib, inhibit TYK2 enzymes by indirectly altering the shape of the enzyme by binding to a site other than the active site.1 Deucravacitinib selectively inhibits TYK2 by allosterically binding to the pseudokinase domain, blocking the receptor-mediated change necessary to bind to ATP, thus preventing activation and downstream signaling of IL-23, IL-12 and type 1 IFNs (Figure 1).1 Orthosteric inhibitors, including brepocitinib and ropsacitinib, compete with ATP for the binding to the active site of the catalytic domains (Figure 1).1
Figure 1. Overview of allosteric and orthosteric inhibition of TYK2*
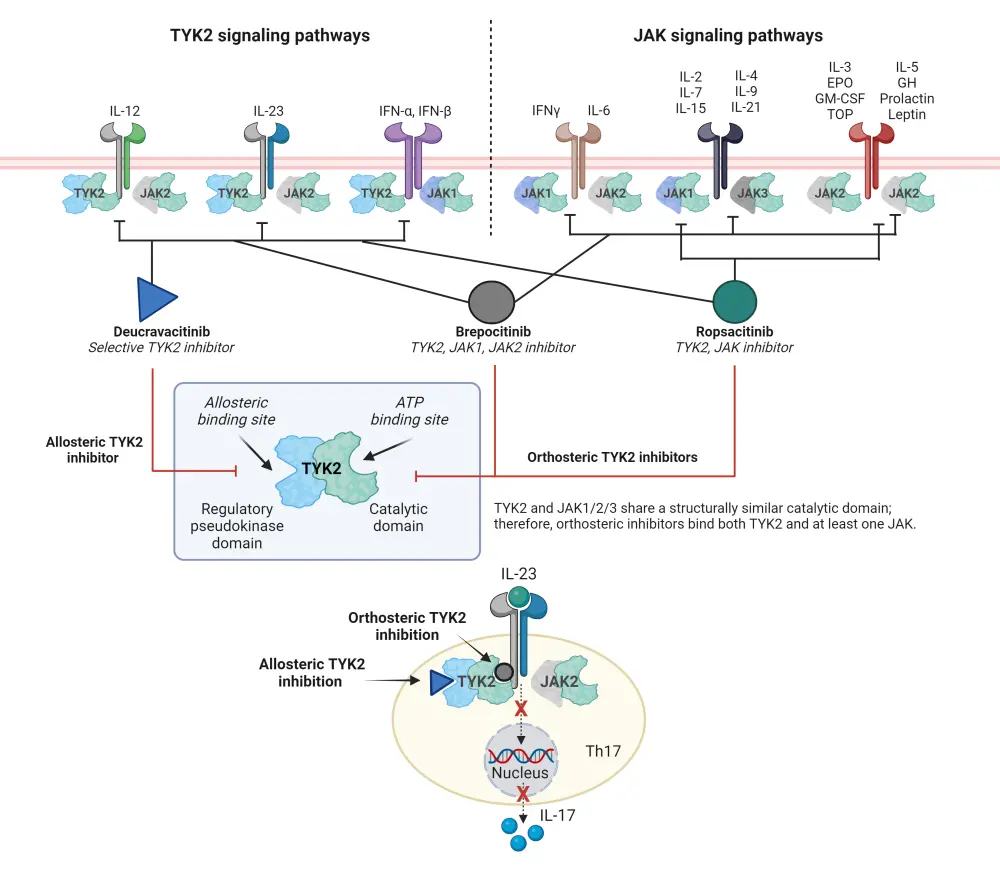
ATP, adenosine triphosphate; EPO, erythropoietin; GH, growth hormone; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; JAK, Janus kinase; Th, helper T cell; TNF, tumor necrosis factor; TPO, thrombopoietin; TYK2, tyrosine kinase 2.
*Adapted from Martin.1 Created with BioRender.com.
Efficacy and safety
Deucravacitinib
Deucravacitinib is the most studied TYK2 inhibitor in patients with psoriasis, with results published from phase II, phase III, and long-term extension (LTE) trials.6–9
Phase II trial (NCT02931838)
A phase II trial (NCT02931838) assessed the safety and efficacy of deucravacitinib at five different doses in adult patients with moderate-to-severe plaque psoriasis.6 In total, 268 patients were randomized 1:1:1:1:1:1 to receive oral deucravacitinib at either 3 mg every other day, 3 mg daily, 3 mg twice daily, 6 mg twice daily, or 12 mg twice daily, or placebo.6 The primary endpoint was met: at Week 12, oral deucravacitinib 3 mg daily, 3 mg twice daily, 6 mg twice daily, or 12 mg twice daily improved the rate of ≥75% reduction in Psoriasis Area and Severity Index (PASI 75) vs placebo (Figure 2). In total, 7% of patients in the placebo group achieved a static Physician’s Global Assessment score of 0 or 1 (sPGA 0/1) at Week 12 vs 20–76% in the deucravacitinib groups (Figure 2).6 Overall, 51% of patients in placebo group experienced AEs, while 55–80% of patients in the deucravacitinib group experienced AEs (Table 2).6
Figure 2. Efficacy at Week 12 in the phase II trial (NCT02931838) of deucravacitinib*
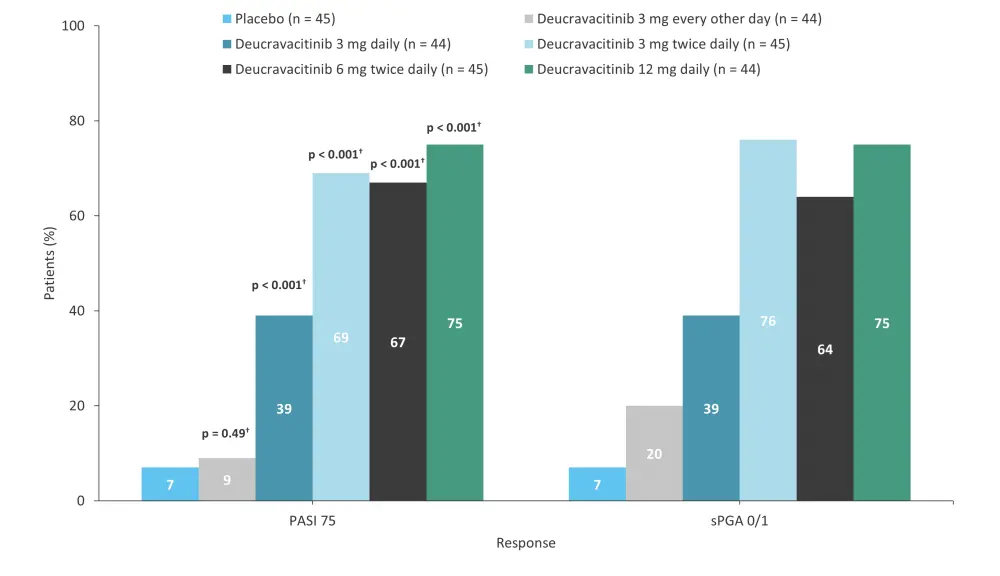
PASI 75, ≥75% reduction in Psoriasis Area and Severity Index; sPGA 0/1, static Physician’s Global Assessment score of 0 or 1.
*Data from Papp, et al.6
†p value vs placebo.
Table 2. AEs in the phase II trial (NCT02931838) of deucravacitinib*
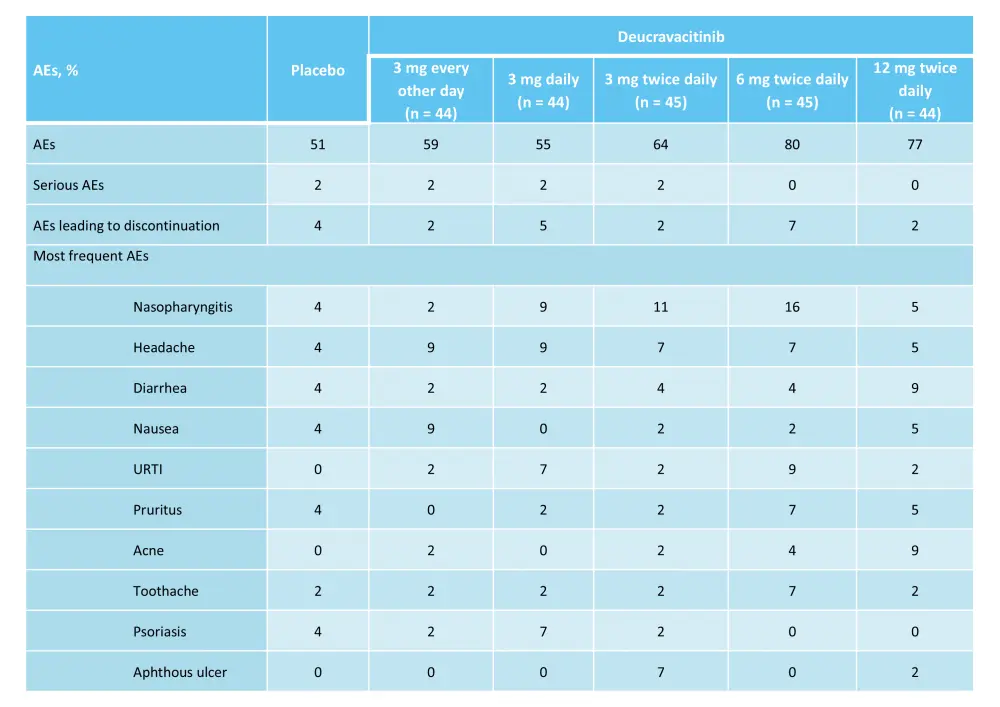
AE, adverse event; URTI, upper respiratory tract infection.
*Adapted from Papp, et al.6
POETYK-PSO-1
The 52-week, randomized, phase III POETYK-PSO-1 trial (NCT03624127) compared the safety and efficacy of deucravacitinib vs apremilast and placebo in adult patients with moderate-to-severe psoriasis.7 In total, 666 patients were randomized 2:1:1 to receive deucravacitinib 6 mg every other day (n = 332), placebo (n = 166), or apremilast 30 mg twice daily (n = 168).7 Response rates for the coprimary endpoints, PASI 75 and sPGA 0/1 with a ≥2-point improvement from baseline at Week 16, were higher with deucravacitinib vs both placebo and apremilast (Figure 3 and Figure 4). The rate of AEs was similar for the three treatment groups (Table 3).7
Figure 3. PASI 75 at Weeks 16 and 24 in the phase III POETYK-PSO-1 trial*
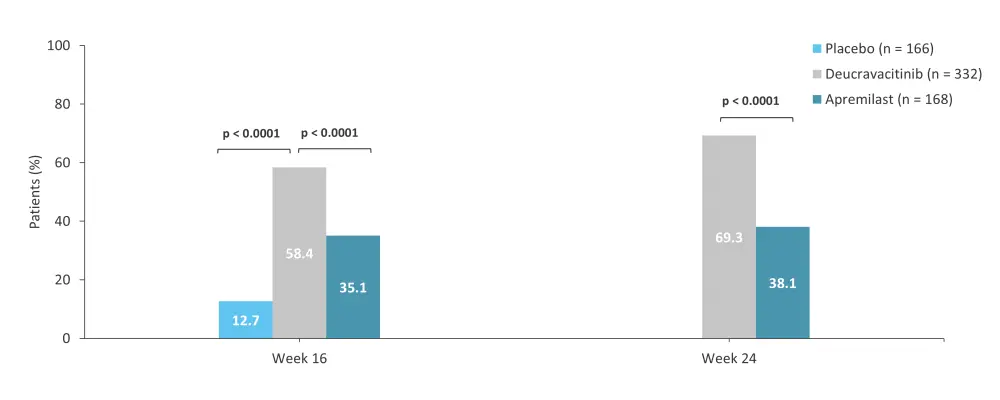
PASI 75, ≥75% reduction in Psoriasis Area and Severity Index.
*Data from Armstrong, et al.7
Figure 4. sPGA 0/1 at Weeks 16 and 24 in the phase III POETYK-PSO-1 trial*
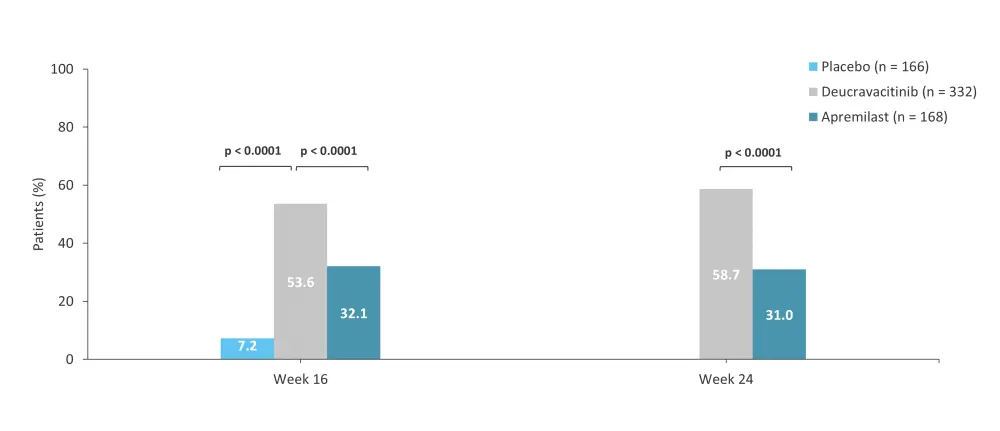
sPGA 0/1, static Physician’s Global Assessment score of 0 or 1.
*Data from Armstrong, et al.7
Table 3. AEs occurring in ≥10% of patients and AEs of interest during Weeks 0–52 in the phase III POETYK-PSO-1 trial*
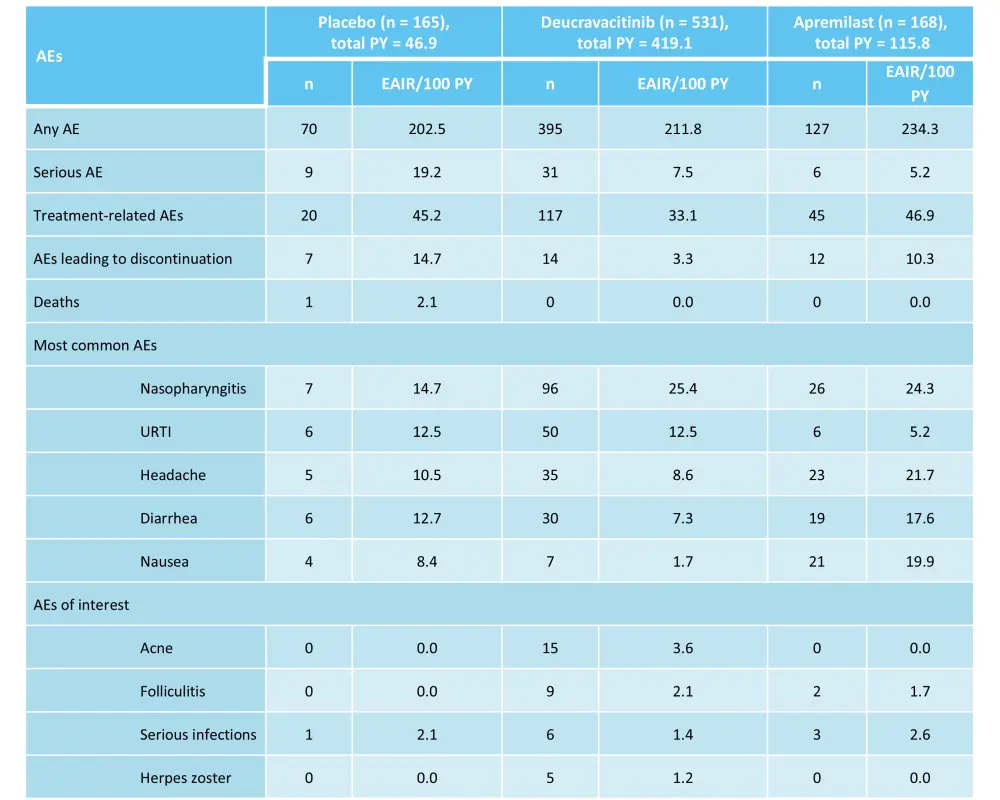
AE, adverse event; EAIR, exposure-adjusted incidence rate; PY, person-years; URTI, upper respiratory tract infection.
*Adapted from Armstrong, et al.7
POETYK-LTE trial
The phase IIIb POETYK-LTE trial (NCT04036435) assessed the long-term outcomes of 1,519 patients who completed the POETYK-PSO-1 and POETYK-PSO-2 trials and continued into the LTE trial.9 Incidences of AEs and SAEs over 2 years are shown in Table 5. Response rates were maintained or improved up to Week 112 (Figure 7).9
Table 5. AEs through 2 years in the POETYK-PSO-1 + POETYK-PSO-2 + POETYK LTE trials*
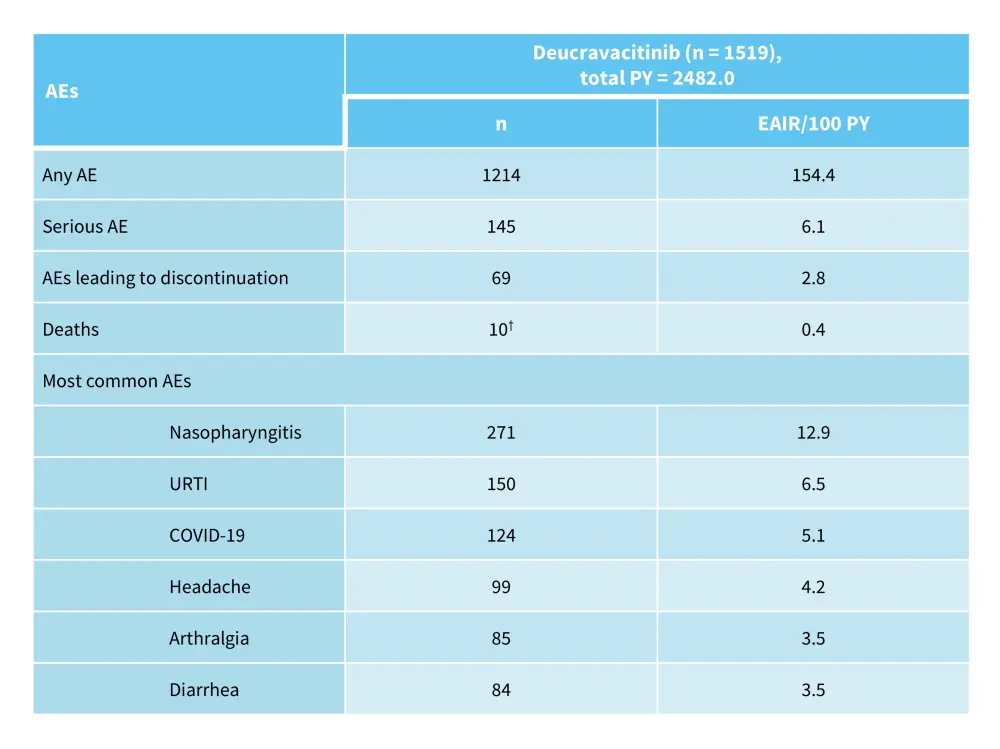
AE, adverse event; EAIR, exposure-adjusted incidence rate; LTE, long-term extension; PY, person-years; URTI, upper respiratory tract infection.
*Adapted from Lebwohl, et al.9
†Includes all deaths regardless of when the last dose was given.
Figure 7. PASI 75, PASI 90, and sPGA 0/1 response rates in patients who continued deucravacitinib treatment until Week 112 in the POETYK-PSO-1 + POETYK-PSO-2 + POETYK LTE trials*
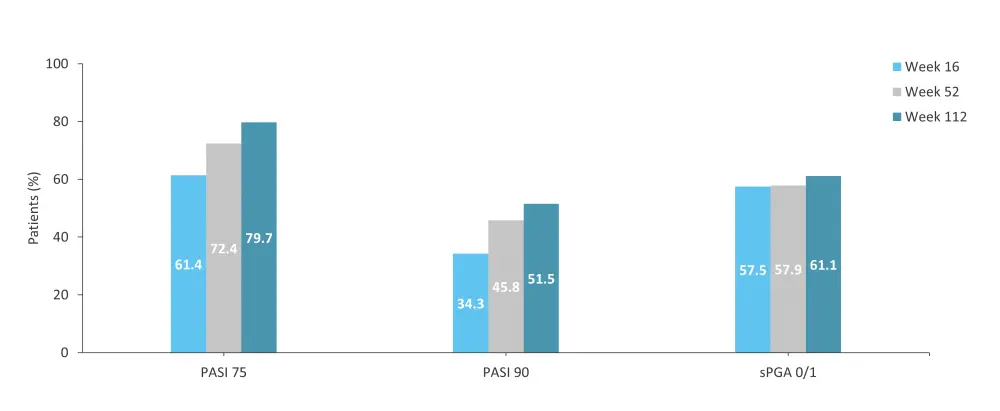
LTE, long-term extension; PASI 75, ≥75% reduction in Psoriasis Area and Severity Index; PASI 90, ≥90% reduction in Psoriasis Area and Severity Index; sPGA 0/1, static Physician’s Global Assessment score of 0 or 1.
*Adapted from Lebwohl, et al.9
During the 25th World Congress of Dermatology 2023, the Psoriasis and Psoriatic Arthritis Hub spoke to Mark Lebwohl, Mount Sinai, New York, U.S., about the long-term efficacy of deucravacitinib.
Long-term efficacy of deucravacitinib
Moderate-to-severe scalp psoriasis
A subgroup analysis of patients in the POETYK-PSO-1 and POETYK-PSO-2 trials with moderate-to-severe scalp psoriasis (n = 1,084) assessed the impact of deucravacitinib on scalp-specific Physician Global Assessment score of 0 or 1 (ss-PGA 0/1) and ≥90% improvement from baseline in Psoriasis Scalp Severity Index (PSSI 90) vs placebo or apremilast.10 At Week 16, a higher proportion of patients treated with deucravacitinib achieved ss-PGA 0/1 vs placebo and vs apremilast (64.0% vs 17.3% vs 37.7%; p < 0.0001).10 Additionally, deucravacitinib was associated with a higher rate of PSSI 90 at Week 16 vs placebo vs apremilast (50.6% vs 10.5% vs 26.1%; p < 0.0001).10 Responses were maintained through 52 weeks with continuous deucravacitinib, and the safety profile was consistent with the entire population.10
Brepocitinib
A phase IIa trial (NCT02969018) investigated the safety and efficacy of brepocitinib in adult patients with moderate-to-severe plaque psoriasis.11 In total, 212 patients were randomized 7:1 to receive 4-week induction with brepocitinib 30 mg, 60 mg daily, or placebo, followed by 8-week maintenance with brepocitinib 10 mg daily, 30 mg daily, or 100 mg once weekly, or placebo.11 At Week 4, the decrease from baseline PASI score was similar between the 30 mg and 60 mg doses; however, at Week 12, patients in the 60 mg → 30 mg daily, 60 mg daily → 100 mg once weekly, 30 mg daily, 30 mg → 10 mg daily, and 30 mg daily → 100 mg once weekly treatment groups had a significant reduction in PASI scores vs placebo.11 Patients in the 30 mg daily treatment group had the greatest reduction in PASI scores from baseline, with a least squares mean change of −17.3, and were more likely to achieve PASI 75 (86.2%) and PASI 90 (51.7%) at Week 12.11 Treatment-emergent AEs and serious AEs, were experienced by 136 and 5 patients treated with brepocitinib, respectively (Figure 8).11
Ropsacitinib
A phase IIb trial (NCT03895372) assessed the safety and efficacy of ropsacitinib in 178 adult patients with moderate-to-severe plaque psoriasis.12 Patients were randomized 1:1:2:2:2 to receive oral ropsacitinib once daily at 50 mg, 100 mg, 200 mg, or 400 mg, or placebo.12 At Week 16, more patients in the 200 mg (risk difference percent, 33.0; 95% confidence interval [CI], 18.0–47.1; p = 0.0004) and 400 mg (risk difference percent, 46.5; 95% CI, 30.6–60.6; p < 0.0001) treatment groups achieved PASI 90 vs placebo.12 The proportion of patients with AEs was similar between ropsacitinib treatment groups but numerically higher vs placebo (Figure 9).12
Figure 8. AEs by brepocitinib dose in the phase IIa trial (NCT02969018)*
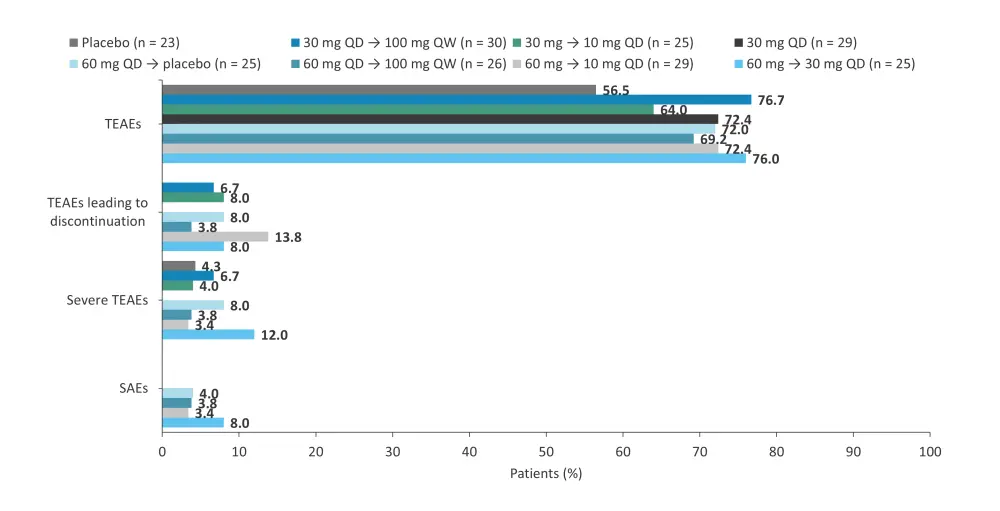
AE, adverse event; QD, once daily; QW, once weekly; SAE, serious AE; TEAE, treatment-emergent AE.
*Data from Forman, et al.11
Figure 9. AEs by ropsacitinib dose in the phase IIb trial (NCT03895372)*
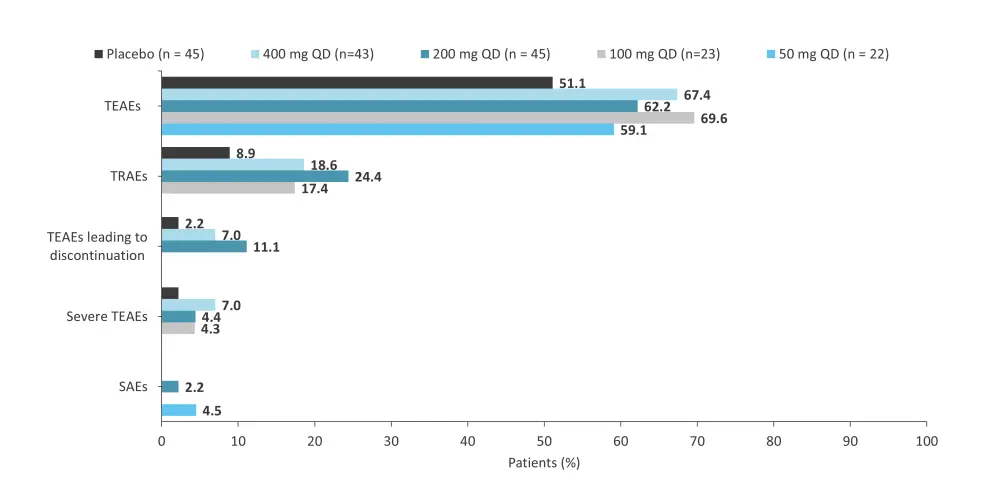
AE, adverse event; QD, once daily; SAE, serious AE; TEAE, treatment-emergent AE; TRAE, treatment-related AE.
*Data from Tehlirian, et al.12
Zasocitinib
A phase IIb trial (NCT04999839) evaluated the safety and efficacy of zasocitinib in 259 patients with moderate-to-severe plaque psoriasis.13 The study design is shown in Figure 10. Results from this trial were previously reported by the Psoriasis and Psoriatic Arthritis Hub. Briefly, at Week 12, the PASI 75, PASI 90, PASI 100, and PGA 0/1 response rates were higher in patients treated with ≥5 mg zasocitinib vs placebo (Figure 11).13 In total, AEs were reported in 53–62% of patients in the treatment arms vs 44% in the placebo arm (Figure 12).
Another phase IIb trial (NCT05153148) assessed the safety and efficacy of zasocitinib in 290 adult patients with active psoriatic arthritis.14 Patients were randomized 1:1:1:1 to receive zasocitinib 5 mg, 15 mg, or 30 mg once daily, or placebo over 12 weeks.14 The primary endpoint was a 20% improvement in the American College of Rheumatology score (ACR 20) at Week 12.14 At Week 12, patients who received zasocitinib 15 mg and 30 mg achieved higher ACR 20 response rates vs placebo (Figure 13).14 The most common treatment-emergent AEs in patients treated with zasocitinib were nasopharyngitis, upper respiratory tract infection, headache, and rash.14 The rates of Grade ≥3 treatment-emergent AEs and serious treatment-emergent AEs were not higher at the 30 mg dose of zasocitinib (Table 6).
Figure 10. Study design of the phase IIb (NCT04999839) trial of zasocitinib*
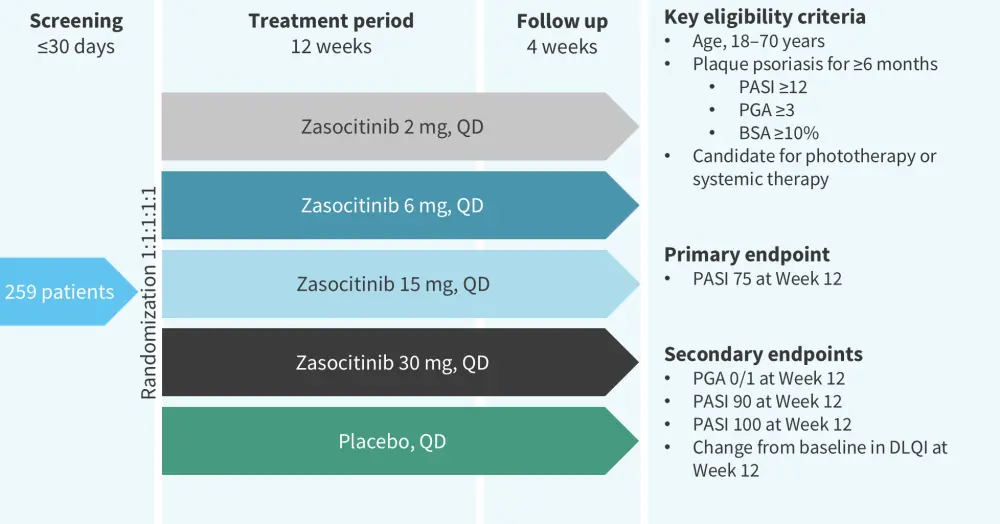
BSA, body surface area; DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index; PGA, Physician’s Global Assessment; QD, once daily.
*Adapted from Armstrong, et al.13
Figure 11. Efficacy at Week 12 in the phase IIb (NCT04999839) trial of zasocitinib*
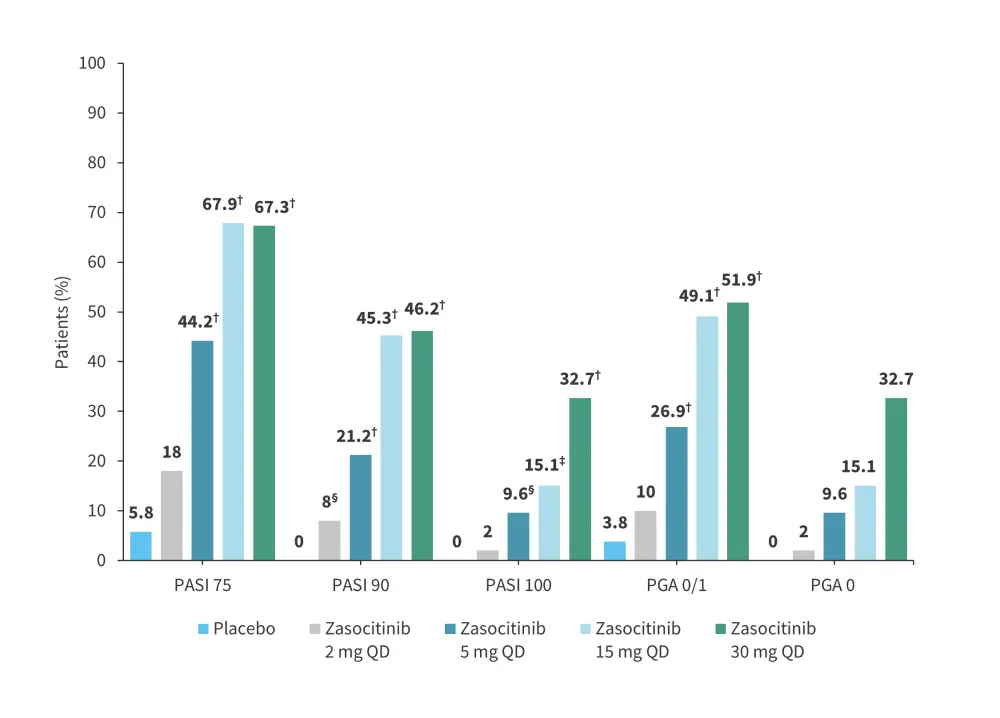
PASI, Psoriasis Area and Severity Index; PGA, Physician’s Global Assessment; QD, once daily.
*Adapted from Armstrong, et al.13
†p < 0.001.
‡p < 0.005.
§p < 0.05.
Figure 12. A The frequency of AEs and B type of AEs according to treatment arm in the phase IIb (NCT04999839) trial of zasocitinib*
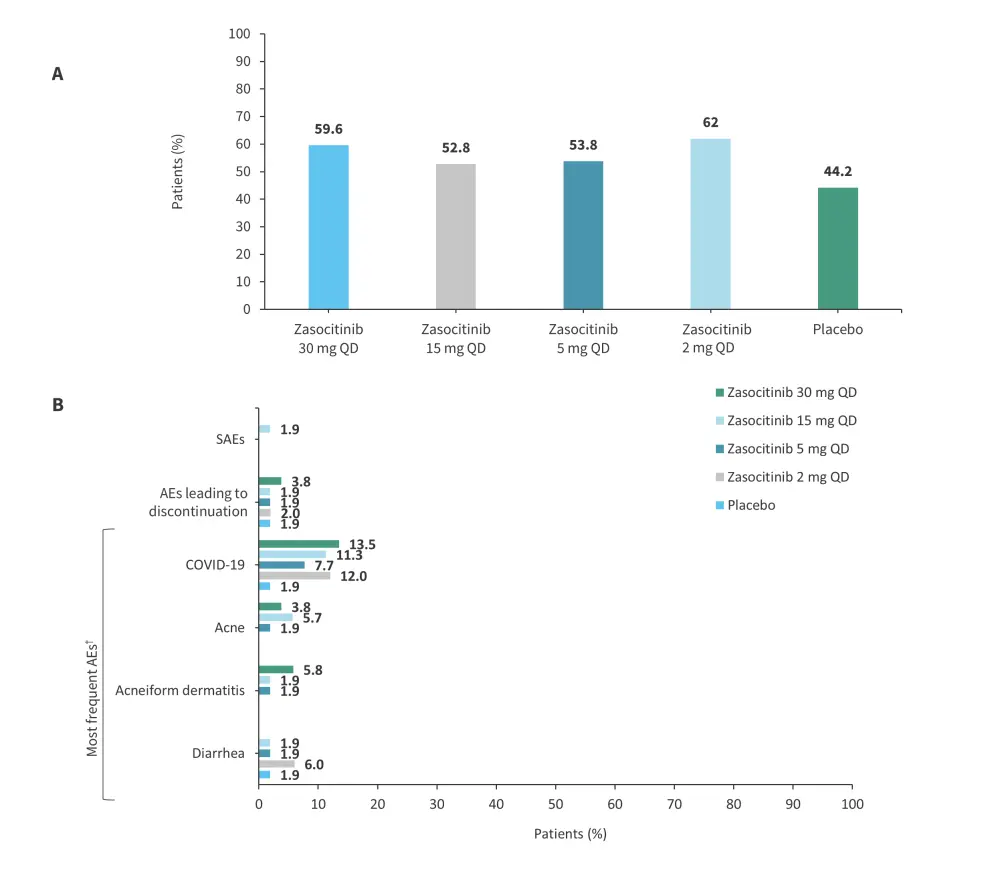
AE, adverse event; QD, once daily; SAE, serious AE.
*Adapted from Armstrong, et al.13
†AEs reported by ≥3 patients in any treatment group (events elicited by laboratory testing are not included).
Figure 13. Efficacy at Week 12 in the phase IIb trial (NCT05153148) of zasocitinib*
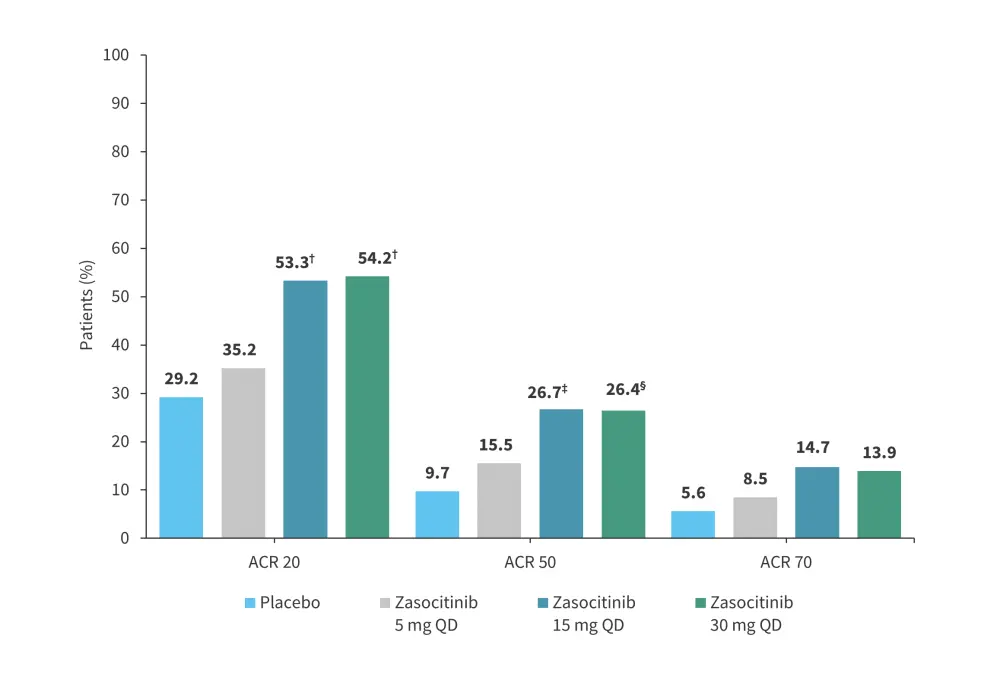
ACR, American College of Rheumatology.
*Adapted from Kivitz.14
†p = 0.002 vs placebo.
‡p = 0.005 vs placebo.
§p = 0.009 vs placebo.
Table 6. AEs reported in the phase IIb trial (NCT05153148) of zasocitinib*
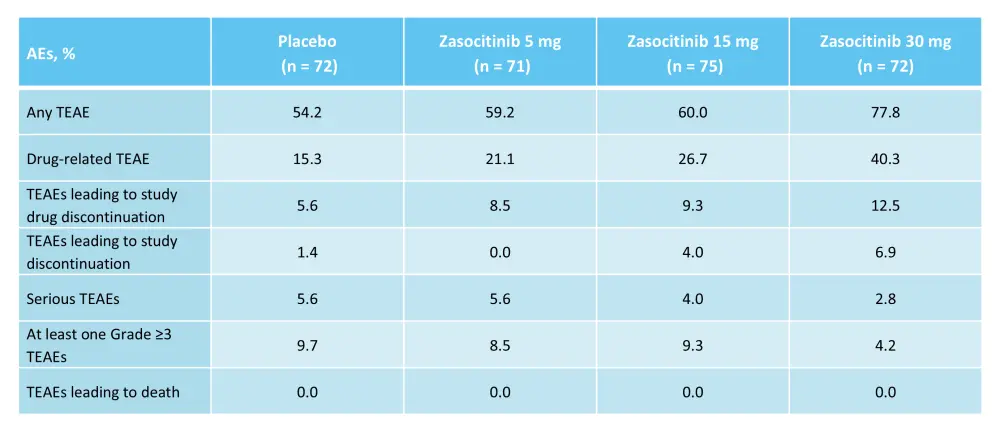
AE, adverse event; TEAE, treatment-emergent AE.
*Adapted from Kivitz.14
Impact on clinical practice
As detailed above, oral inhibitors that target the JAK/STAT pathway have demonstrated efficacy in patients with psoriasis.4 Oral therapies offer advantages over intravenous or subcutaneous therapies, with less risk of immunogenicity and infection site reactions, easier transportation and storage, and improved patient adherence and QoL.1,4 TYK2 inhibitors, in particular, have an advantage over JAK inhibitors due to their higher selectivity and reduced potential for off-target effects.4 In addition, the potential for improved safety, efficacy, and the convenience of oral TYK2 inhibitors suggest that this is a promising new class of therapeutics for patients with psoriasis.
In a network meta-analysis of 47 randomized controlled studies by Armstrong et al. that compared deucravacitinib to other approved systemic treatments for patients with moderate-to-severe plaque psoriasis, deucravacitinib was associated with similar long-term efficacy to some biologics, including etanercept, infliximab, secukinumab, adalimumab, and ustekinumab.15 The short-term analysis (10–16 weeks) and mid-term analysis (24–28 weeks) found that deucravacitinib gave a higher estimated PASI 75 response rate vs etarnercept 50 mg, methotrexate, etanercept 25 mg, and apremilast, and was within the range of first-generation biologics, while in the long-term analysis (44–60 weeks), PASI 75 response rates for deucravacitinib were higher than methotrexate, apremilast, etanercept 50 mg, and infliximab, and were similar to secukinumab, adalimumab, and ustekinumab (Figure 14).
Figure 14. Estimated long-term (44–60 weeks) PASI 75 response in a network meta-analysis of 47 trials*
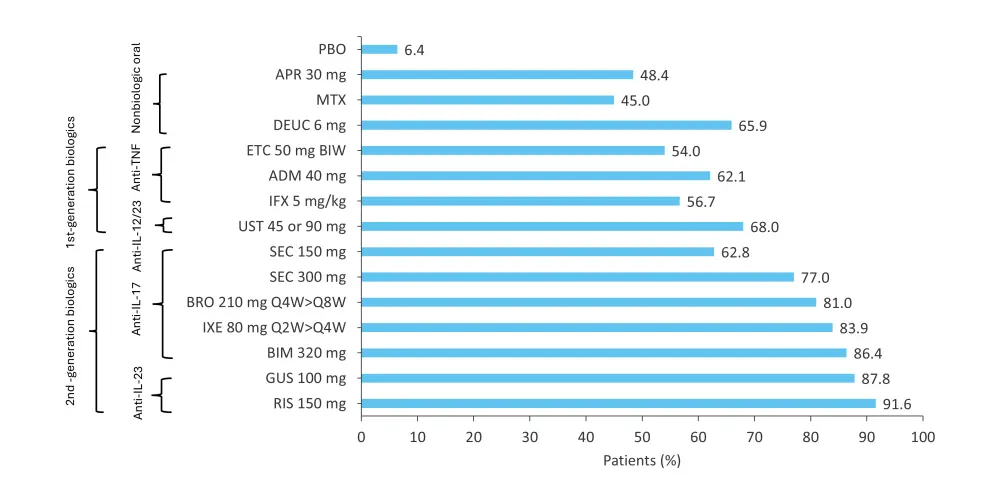
ADM, adalimumab; APR, apremilast; BIM, bimekizumab; BIW, twice weekly; BRO, brodalumab; DEUC, deucravacitinib; ETC, etanercept; GUS, guselkuma; IFX, infliximab; IL, interleukin; IXE, ixekizumab; MTX, methotrexate; PASI 75, ≥75% reduction in Psoriasis Area and Severity Index; PBO, placebo; Q2W, once every 2 weeks; Q4W, once every 4 weeks; Q8W, once every 8 weeks; RIS, risankizumab; SEC, secukinumab; TNF, tumor necrosis factor; UST, ustekinumab.
*Adapted from Armstrong, et al.15
Question 1 / 1
What is the primary method of administration for TYK2 inhibitors?
A
Subcutaneous
B
Oral
C
Topical
D
Intravenous
Furthermore, a network meta-analysis by Xu et al. that included 13 randomized controlled trials assessed the benefit and risk profile of oral TYK2 and phosphodiesterase 4 (PDE4) inhibitors in patients with moderate-to-severe plaque psoriasis.3 In this analysis, deucravacitinib 3 mg twice daily, 6 mg once daily, 6 mg twice daily, 12 mg once daily, and ropsacitinib 400 mg once daily were associated with higher rates of PASI 75 than apremilast 30 mg twice daily.3 Efficacy ranking analysis demonstrated that patients treated with deucravacitinib 12 mg once daily were more likely to achieve PASI 75 (SUCRA value: 0.946) followed by deucravacitinib 3 mg twice daily (SUCRA value: 0.887), deucravacitinib 6 mg twice daily (SUCRA value: 0.863) and ropsacitinib 400 mg once daily (SUCRA value: 0.835).3 In the safety ranking analysis, apremilast 10 mg twice daily was associated with the lowest probability of AE occurrence (SUCRA value: 0.872), followed by deucravacitinib 3 mg once daily (SUCRA value: 0.692), and ropsacitinib 50 mg once daily (SUCRA value: 0.649).3 Taken together, these results highlight the potential benefit of TYK2 inhibitors, particularly deucravacitinib, over other therapies.3,15
Question 1 / 1
Which of the following treatments would you expect to give the highest likelihood of achieving PASI 75 in patients with moderate-to-severe plaque psoriasis?
A
Deucravacitinib 6 mg twice daily
B
Deucravacitinib 12 mg once daily
C
Ropsacitinib 400 mg once daily
D
Deucravacitinib 3 mg twice daily
Future directions
Several studies are ongoing to assess the safety and efficacy of deucravacitinib in patients with psoriatic arthritis (POETYK PsA-2, NCT04908189), palmoplantar pustulosis (NCT05710185), moderate-to-severe scalp psoriasis (PSORIATYK SCALP, NCT05478499), nail psoriasis (NCT05124080), and pediatric psoriasis (NCT04772079).2 However, further studies are also warranted to determine the long-term efficacy of TYK2 inhibitors to optimize the selection of patients for these therapies and to compare the safety profile with JAK inhibitors.4
Conclusion
A better understanding of the pathogenesis of psoriasis has led to the development of TYK2 inhibitors, an efficacious new oral therapeutic option for patients with psoriasis.1 Long-term results may further validate the safety and efficacy benefit of TYK2 inhibitors, and future studies could identify factors associated with response to therapy.1,2
Your opinion matters
As a result of this content, I commit to reviewing efficacy and safety findings for tyrosine kinase 2 inhibitors to guide my treatment of patients with psoriasis in clinical practice.
This educational resource is independently supported by Bristol Myers Squibb. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

