All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
NDI-034858 (TAK-279) in moderate-to-severe plaque psoriasis: Results from a phase IIb trial
Tyrosine kinase 2 (TYK2) is a key component of the Janus kinase/signal transducer activator transcription (JAK/STAT) signaling pathway that regulates intracellular signaling of several cytokines, including interleukin-12/-23, as well as type I interferons, implicated in the pathophysiology of psoriasis.1,2 Hence, selective TYK2 inhibition may be a promising therapeutic alternative in the treatment of psoriasis.1,2
NDI-034858 (TAK-279) is a novel, investigational, oral, allosteric TYK2 inhibitor and is highly selective to TYK2 owing to a single amino acid difference from TYK2 that prevents it from binding to the allosteric pocket of JAK1.1,2 Preliminary evidence of the effect of TAK‑279 on psoriasis has been demonstrated in a phase I trial.2
At the American Academy of Dermatology (AAD) Annual Meeting 2023, Armstrong1 presented the efficacy and safety results of the phase IIb study (NCT04999839) of TAK-279 in patients with moderate-to-severe plaque psoriasis. Here, we summarize the key findings.
Study design1
The phase IIb randomized, multicenter, double-blind, placebo-controlled study was designed to assess the efficacy, safety, and tolerability of TAK-279 in adult patients with moderate-to-severe plaque psoriasis. Patients were randomized (1:1:1:1:1 ratio) to receive one of four doses of TAK-279 (2 mg, 5 mg, 15 mg, or 30 mg once daily [QD]) or the placebo for 12 weeks. The detailed study design is illustrated in Figure 1.
Figure 1. Study design*
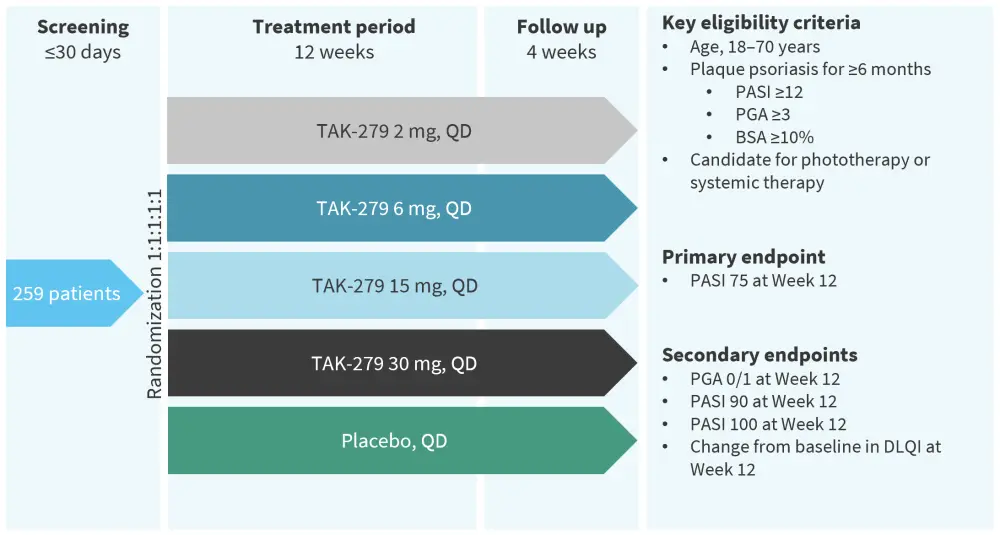
BSA, body surface area; DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index; PGA, Physician’s Global Assessment; QD, once daily.
*Adapted from Armstrong, et al.1
Results1
A total of 259 patients with moderate-to-severe plaque psoriasis were included in the study. The key baseline patient characteristics are shown in Table 1. Across the treatment arms, the mean age was between 45 years and 49 years and most patients (≥60%) were male.
Table 1. Demographics and baseline patient characteristics*
|
AA, African American; BSA, body surface area; DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index; PGA, Physician’s Global Assessment; QD, once daily; SD, standard deviation. |
|||||
|
Characteristic, mean (SD) (unless otherwise stated) |
Placebo |
TAK-279 |
TAK-279 |
TAK-279 |
TAK-279 |
|---|---|---|---|---|---|
|
Age, years |
48.8 (12.7) |
45.8 (14.2) |
45.1 (13.6) |
46.2 (13.0) |
48.5 (11.4) |
|
Male, % |
59.6 |
76.0 |
78.8 |
64.2 |
63.5 |
|
White, % |
84.6 |
86.0 |
76.9 |
86.8 |
80.8 |
|
Psoriasis duration, years |
12.7 (10.5) |
13.8 (10.8) |
14.8 (10.7) |
17.6 (14.6) |
17.4 (11.1) |
|
PASI score |
18.3 (8.1) |
18.4 (6.8) |
18.6 (6.1) |
15.5 (4.5) |
17.6 (6.2) |
|
PGA score |
3.2 (0.4) |
3.4 (0.5) |
3.3 (0.5) |
3.2 (0.4) |
3.2 (0.4) |
|
BSA |
21.3 (13.6) |
24.9 (15.5) |
22.6 (12.1) |
18.3 (10.3) |
22.2 (14.3) |
|
DLQI |
12.4 (7.0) |
10.3 (6.2) |
12.8 (7.5) |
11.9 (7.1) |
12.5 (6.9) |
|
Bioexperienced, % |
8 (15.4) |
8 (16.0) |
15.4 |
17.0 |
15.4 |
Efficacy
The primary endpoint was met, with a significantly greater proportion of patients treated with ≥5 mg TAK-279 achieving PASI 75 versus placebo at Week 12 (Figure 2).
A significantly greater proportion of patients receiving ≥5 mg TAK-279 achieved PASI 90, PASI 100, and PGA 0/1 (almost clear skin) versus placebo. In the TAK-279 30 mg QD arm, ~33% of the patients scored PGA 0, indicating totally clear skin. There were no statistically significant differences in PASI 75, PASI 100, or PGA response rates in the TAK-279 2 mg arm compared with placebo (Figure 2).
Figure 2. NRI analysis: Patients achieving primary and secondary endpoints at Week 12*
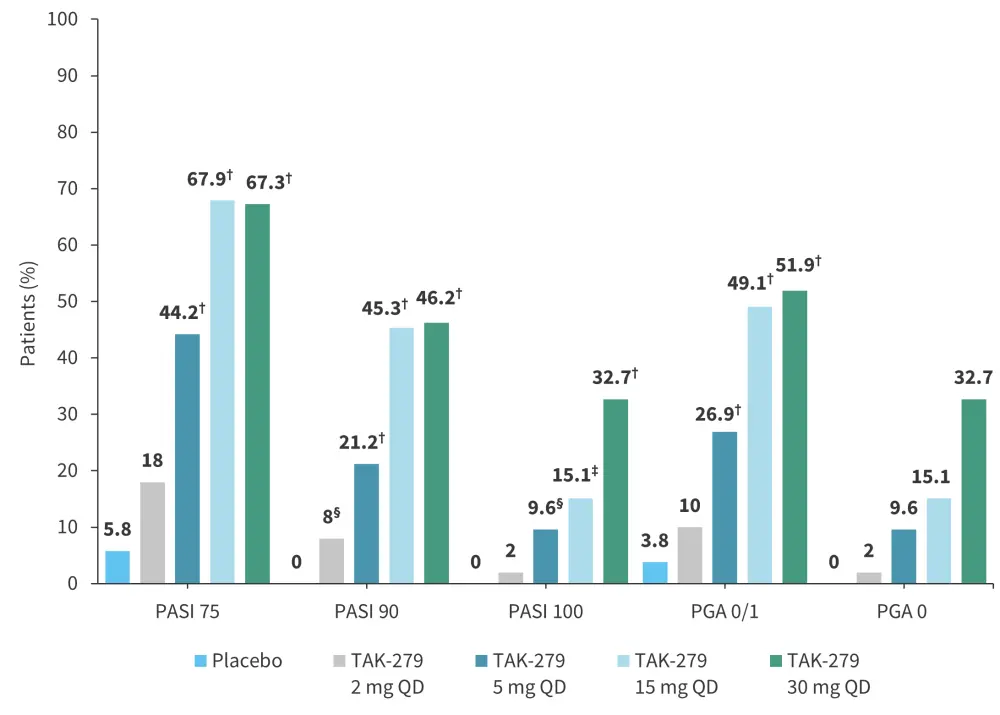
NRI, non-responder imputation; PASI, Psoriasis Area and Severity Index; PGA, Physician’s Global Assessment; QD, once daily.
*Adapted from Armstrong, et al.1
†p < 0.001.
‡p < 0.005.
§p < 0.05.
Safety
The frequency of adverse events (AEs) was 53–62% in the treatment arms and 44% in the placebo arm (Figure 3A). Two serious AEs occurred in one patient on TAK-279 15 mg and were considered unrelated to the treatment (Figure 3B). The most common treatment-emergent AEs (TEAEs) were COVID-19, acne, acneiform dermatitis, and diarrhea. Few patients with TEAEs (1–2 per treatment group) discontinued the treatment. No deaths were reported.
Figure 3. Safety findings: A The frequency of AEs and B type of AEs according to treatment arm*
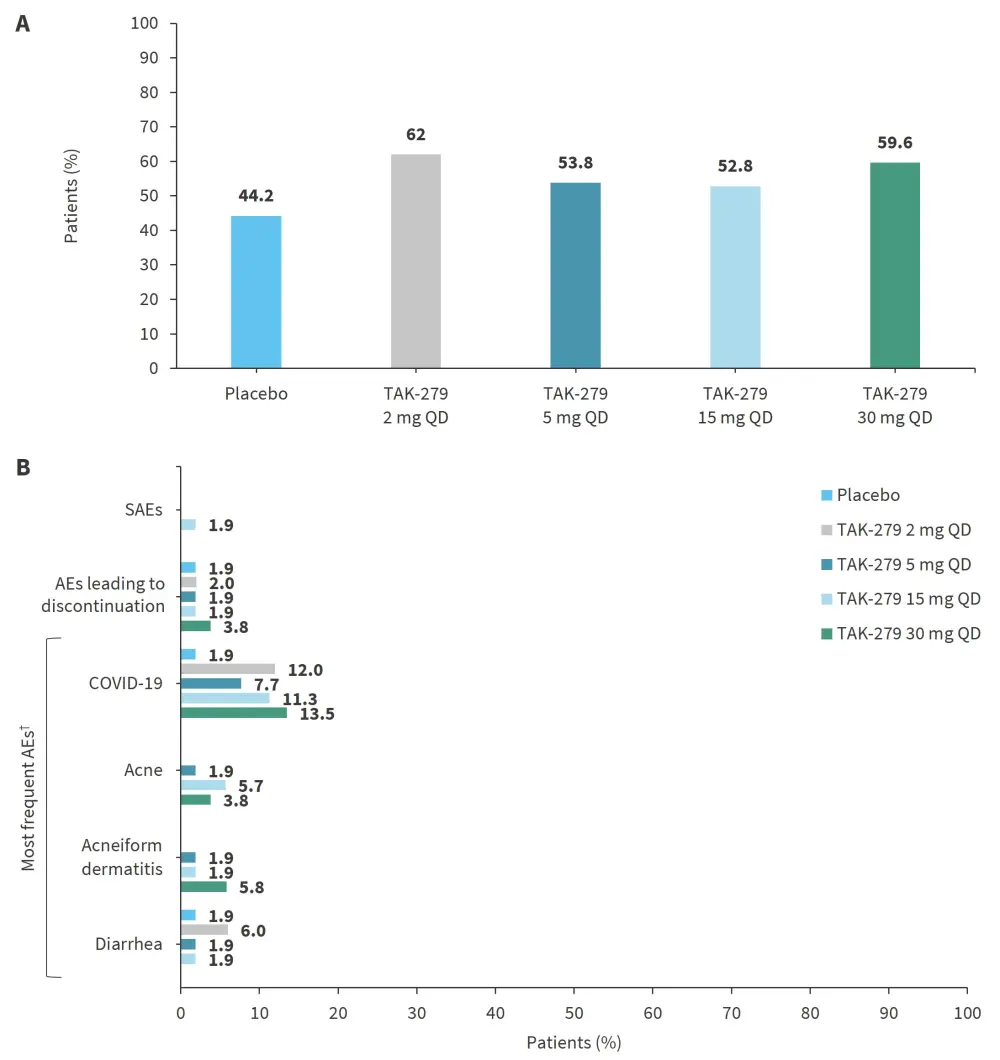
AE, adverse event; QD, once daily; SAE, serious AE.
*Adapted from Armstrong, et al.1
†AEs reported by ≥3 patients in any treatment group (events elicited by laboratory testing are not included).
The laboratory parameter changes were consistent with TYK2 inhibition. There were no adverse trends for hematologic (cell counts or creatine phosphokinase levels), hepatic and renal (liver enzymes, creatinine, or estimated glomerular filtration rate), or lipid parameters (cholesterol, high-density lipoprotein, or low-density lipoprotein levels).
Conclusion
The study achieved its primary and secondary endpoints, with a significantly greater proportion of patients receiving TAK-279 achieving PASI 75, 90, and 100 and PGA 0/1 at ≥5 mg dose groups compared with placebo at 12 weeks. The phase IIb findings support further larger studies of TAK-279 in psoriasis.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

