All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Secukinumab for plaque psoriasis: Results from the 4-year extension of ERASURE and FIXTURE
Do you know... The mean Psoriasis Area and Severity Index (PASI) score at the end of Year 1 of treatment with secukinumab was 1.84 (as observed), representing a 91.8% decrease from baseline. What was the mean PASI score (as observed) at Year 5 of treatment?
Secukinumab, an anti-IL-17A monoclonal antibody, is recommended at a dose of 300 mg in patients with plaque psoriasis.1 The safety and efficacy of secukinumab has previously been confirmed in the phase III SCULPTURE trial (NCT01406938). More recently, the phase III ERASURE (NCT01365455) and FIXTURE (NCT01358578) trials investigated the safety and efficacy of secukinumab at two different doses to treat moderate-to-severe plaque psoriasis.1 Here, we discuss data from the combined extension phase of ERASURE and FIXTURE up to 5 years from trial initiation.
Study design1
In the double-blind core studies of ERASURE and FIXTURE (up to Year 1), patients were randomized to receive either 300 mg or 150 mg of secukinumab. A total of 1,366 patients completed the core trials to Year 1, with 1,146 entering the extension; 934 patients completed up to Year 3 of the extension study, with 883 patients entering the open-label period; and 777 completed up to Year 5, the end of the extension phase. The study design of the ERASURE–FIXTURE extension study is shown in Figure 1.
Figure 1. ERASURE and FIXTURE core and extension design*
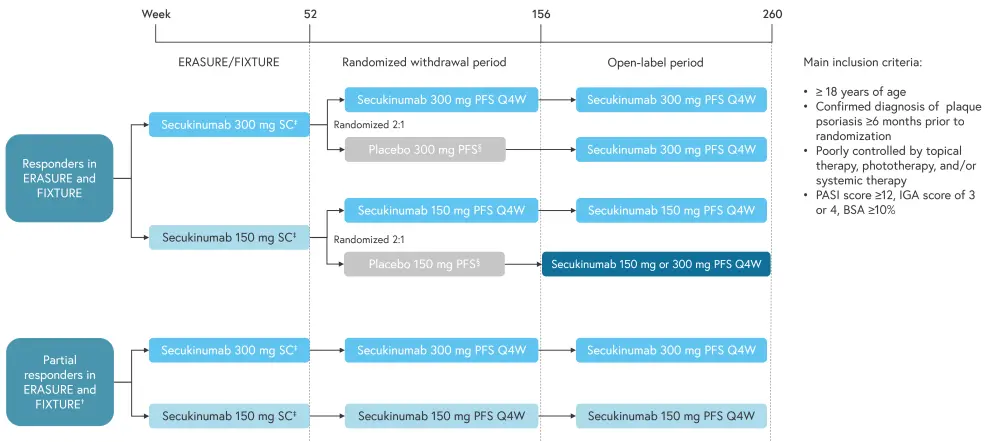
IGA, Investigator Global Assessment; PASI, Psoriasis Area and Severity Index; PFS, pre-filled syringe; Q4W, every four weeks; SC, subcutaneous.
*Adapted from Langley, et al.1
†Partial response defined as PASI 50 responders.
‡In the core trials, patients received secukinumab 150 mg or 300 mg weekly from baseline to Week 4, then every 4 weeks to Week 48.
§Patients on placebo who relapsed were returned to secukinumab treatment.
Results1
In the core trials, the majority of patients were male and had achieved a 75% improvement in Psoriasis Area and Severity Index (PASI 75) from baseline. Patient characteristics at baseline of the extension phase are shown in Table 1.
Table 1. Patient characteristics in randomized cohort in extension*
|
BMI, body mass index; IGA, Investigator Global Assessment; PASI, Psoriasis Area and Severity Index. |
|
|
Characteristic, % (unless otherwise stated) |
All patients (N = 1,146) |
|---|---|
|
Mean age, years |
46.2 |
|
Male |
71.2 |
|
Mean bodyweight at start of extension, kg |
86.9 |
|
Mean BMI at start of extension |
29.3 |
|
Mean PASI score at start of extension |
2.1 |
|
PASI response at start of extension |
|
|
<PASI 50 |
0.1 |
|
≥PASI 50 |
99.9 |
|
≥PASI 75 |
86.4 |
|
≥PASI 90 |
66.1 |
|
IGA score at start of extension |
|
|
3 |
8.1 |
|
4 |
0.3 |
Safety
The median duration of secukinumab exposure for both doses was 1,438 days. Safety analysis did not reveal an increased incidence of adverse events over the 5 years; the overall incidence rates of adverse events throughout the entire extension period are shown in Table 2. There were two deaths reported, both were not considered treatment related.
Table 2. Adverse events across the entire study period*
|
AE, adverse event; EAIR, exposure-adjusted incidence rate; MACE, major acute cardiovascular event. |
|
|
Event, EAIR per 100 patient years |
All years (n = 890) |
|---|---|
|
All AEs |
140 |
|
Common AEs† |
|
|
Infections and infestations |
61.7 |
|
Nasopharyngitis |
14.2 |
|
Upper respiratory tract infection |
5.1 |
|
Deaths |
0.1 |
|
Serious AEs |
5.7 |
|
AEs leading to discontinuation |
1.2 |
|
AEs of special interest |
|
|
Infections |
62.3 |
|
Hypersensitivity |
5.6 |
|
Neutropenia |
0.5 |
|
Malignant or unspecified tumors |
0.5 |
|
MACE |
0.3 |
Efficacy
The proportion of patients treated with secukinumab 300 mg in both the response and partial-response groups achieving PASI 75, PASI 90, and PASI 100 remained consistent from Year 3 to 5, as shown in Figure 2.
Figure 2. Proportion of patients treated with secukinumab 300 mg achieving PASI 75, PASI 90, and PASI 100*
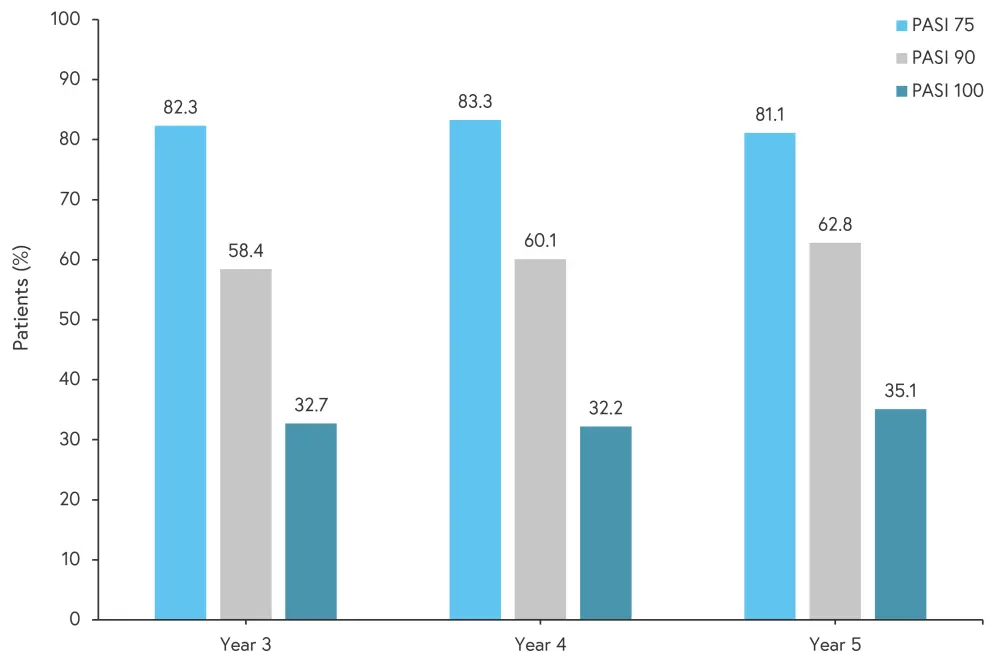
PASI, Psoriasis Area and Severity Index.
*Adapted from Langley, et al.1
The mean PASI score increased from 1.84 to 2.61 from Year 1 to 5 in patients who achieved a response or partial response in the core study and proceeded to receive secukinumab 300 mg in the extension. At Year 1 and 5, this represents a 91.8% and 88.1% decrease in mean PASI score from baseline, respectively. The percentage of patients achieving Investigator’s Global Assessment 0/1 decreased from Year 1 to Year 5, as illustrated in Figure 3.
Figure 3. A Mean PASI score in patients treated with secukinumab 300 mg and B the percentage of patients treated with secukinumab 300 mg who achieved IGA 0/1*
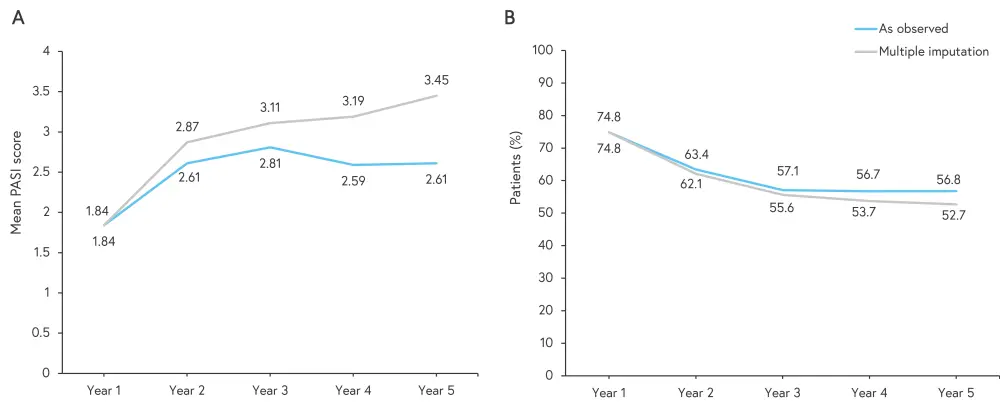
IGA, Investigator’s Global Assessment; PASI, Psoriasis Area and Severity Index.
*Adapted from Langley, et al.1
Quality of life measures
Using Dermatology Life Quality Index (DLQI) measurements, quality of life benefits were maintained up to Year 5 of treatment. The mean DLQI score was 1.8 at Year 1, increasing to 2.7 at Year 3, and 3.3 at Year 5. Figure 4 shows the percentage of patients treated with secukinumab 300 mg who achieved a DLQI score of 0 or 1 (the condition is having little or no impact on the quality of life).
Figure 4. Percentage of patients treated with secukinumab 300 mg achieving a DLQI of 0 or 1*
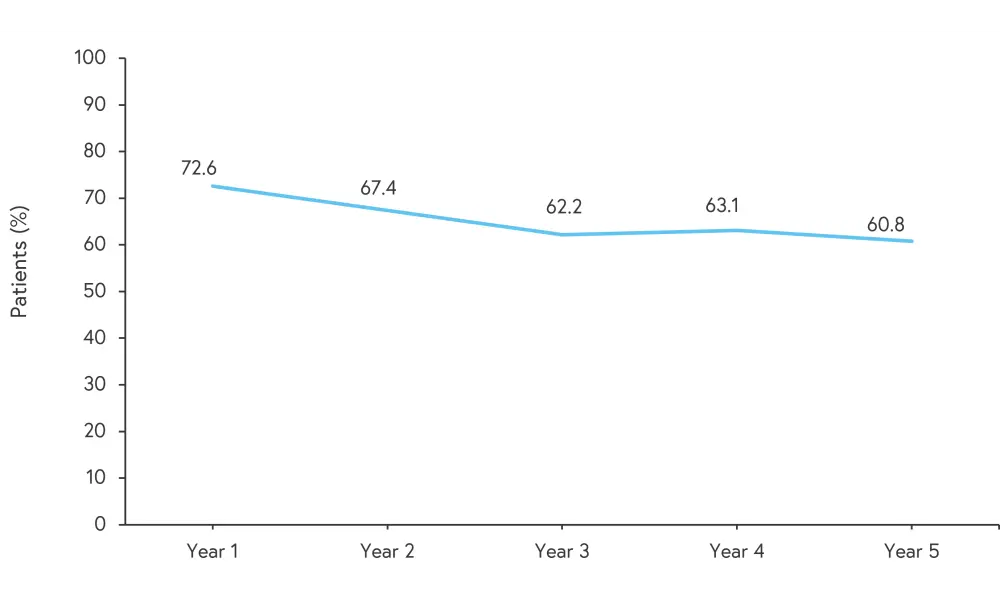
DLQI; Dermatology Life Quality Index.
*Adapted from Langley, et al.1
Conclusion
In the extension study of ERASURE and FIXTURE, secukinumab demonstrated sustained efficacy at 5 years in patients with moderate-to-severe plaque psoriasis; however, loss of response was observed in some patients. In recent data from the FIXTURE and CLEAR (NCT02074982) trials, a bodyweight <90 kg and not having experienced a failed biologic therapy was associated with more durable responses to secukinumab; this could explain a reduced response in this subset of patients. In addition, a higher BMI score has been associated with poorer PASI responses at Week 78 of secukinumab treatment, which may be linked to secukinumab plasma levels; during the FIXTURE trial, there were higher efficacy rates in patients with higher secukinumab plasma levels.
Data from real-world studies is needed to fully elucidate the efficacy and safety of secukinumab; however, these findings may be limited by differences in treatment sequencing, prior biologic exposure, and less rigorous follow-up compared with clinical trials.
The efficacy and safety data reported in these trials were similar to results of the previous SCULPTURE trial, with no additional safety signals observed in the extension phase. For the majority of patients, this suggest that secukinumab could be safely used for prolonged periods while maintaining responses.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?


