All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Week 24 results of a phase III trial in pediatric patients assessing secukinumab for the treatment of plaque psoriasis in children and young people
Psoriasis is a debilitating chronic skin condition, with plaque psoriasis representing the most common form in pediatric patients.1 Despite well-established treatment guidelines for adult patients with psoriasis, treatment options in children are limited, and there are fewer clinical trials in the pediatric and adolescent population than in adults.1
However, in October 2021, the National Institute for Health and Care Excellence (NICE) approved the use of the interleukin (IL)-17A monoclonal antibody secukinumab for the treatment of plaque psoriasis in children and young people aged 6–17 years.2 The guidance recommends secukinumab for patients with moderate-to-severe disease, indicated by a Psoriasis Area and Severity Index (PASI) ≥10, and if the disease has failed to respond to, or the patient is not able to have, existing systemic therapies, including drugs such as ciclosporin and methotrexate, or phototherapy.
Here, we summarize the work of Magnolo et al.1 who reported the 24-week outcomes from their phase III clinical trial of secukinumab in the treatment of pediatric patients with plaque psoriasis (NCT03668613). The study was published in the Journal of Academic Dermatology in January 2022.
Study design and patient characteristics
A phase III, multi-center, open-label, randomized clinical trial, was conducted across 23 sites around the world. A total of 92 patients were recruited, of whom 84 completed the screening phase. These patients were evenly randomized to high dose (HD) and low dose (LD) secukinumab groups. The study design can be seen in Figure 1 and baseline characteristics are detailed in Table 1.
Figure 1. Study design*
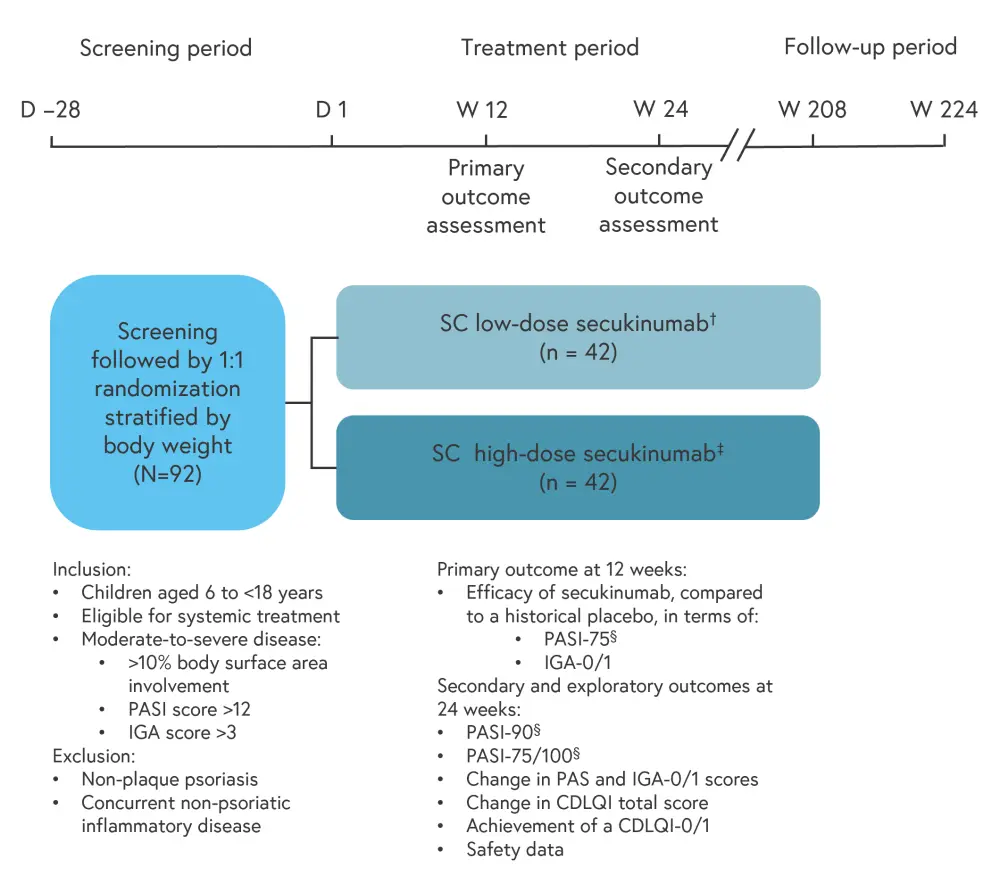
D, day; CDLQI, Children’s Dermatology Quality of Life Index; IGA, Investigator Global Assessment; PASI, Psoriasis Area and Severity Index; SC, subcutaneous; W, week.
*Data from Magnolo, et al.1
†Administered according to body weight: 75 mg for patients weighing <25 kg, 75 mg for patients weighing 25–49 kg, and 150 mg for patients weighing ≥50 kg.
‡Administered according to body weight: 75 mg for patients weighing <25 kg, 150 mg for patients weighing 25–49 kg, and 300 mg for patients weighing ≥50 kg.
§PASI-75/90/100 refers to the number of patients who have achieved a 75%/90%/100% reduction in their PASI score from baseline.
Table 1. Baseline characteristics of study participants*
|
HD, high dose; IGA, Investigator Global Assessment; LD, low dose; PASI, Psoriasis Area and Severity Index. |
||
|
Characteristic, % (unless otherwise stated) |
LD secukinumab |
HD secukinumab |
|---|---|---|
|
Age, years |
|
|
|
6 to <12 |
40.5 |
38.1 |
|
12 to <18 |
59.5 |
61.9 |
|
Mean age, years (SD) |
12.5 (2.39) |
12.8 (3.42) |
|
Female |
47.6 |
59.5 |
|
Disease severity |
|
|
|
Moderate |
71.4 |
73.8 |
|
Severe |
28.6 |
26.2 |
|
Baseline PASI |
|
|
|
≤20 |
64.3 |
57.1 |
|
>20 |
35.7 |
42.9 |
|
Baseline IGA score |
|
|
|
Grade 3 (moderate) |
69.0 |
69.0 |
|
Grade 4 (severe) |
31.0 |
31.0 |
|
Mean weight, kg (SD) |
54.3 (19.7) |
55.7 (19.3) |
|
Weight, n (%) |
|
|
|
<25 kg |
4 (9.5) |
4 (9.5) |
|
25–49 kg |
13 (31) |
12 (28.6) |
|
≥50 kg |
25 (59.5) |
26 (61.9) |
Results
At 12 weeks, predictive log odds ratios (Table 2) suggest that treatment with secukinumab is superior for both the LD and HD groups relative to historical placebo. The estimated probability of a positive treatment effect with secukinumab was 100% when compared with placebo. In addition, both groups demonstrated good response rates in terms of PASI-75, PASI-90, and Investigator Global Assessment (IGA)-0/1 (Figure 2).
Table 2. Predictive log odds ratios of secukinumab at 12 weeks compared with a historical placebo*†
|
HD, high dose; IGA, Investigator Global Assessment; LD, low dose; OR, odds ratio; PASI, Psoriasis Area and Severity Index. |
||
|
Predictive log OR (95% credible interval) |
LD secukinumab |
HD secukinumab |
|---|---|---|
|
PASI-75 |
4.9 (3.4–6.8) |
4.8 (3.4–6.8) |
|
PASI-90 |
4.4 (2.9–6.2) |
4.7 (3.2–6.6) |
|
IGA-0/1 |
4.3 (2.7–6.5) |
4.4 (2.9–6.6) |
Figure 2. Response rates according to PASI and IGA-0/1 scores across the secukinumab treatment groups*
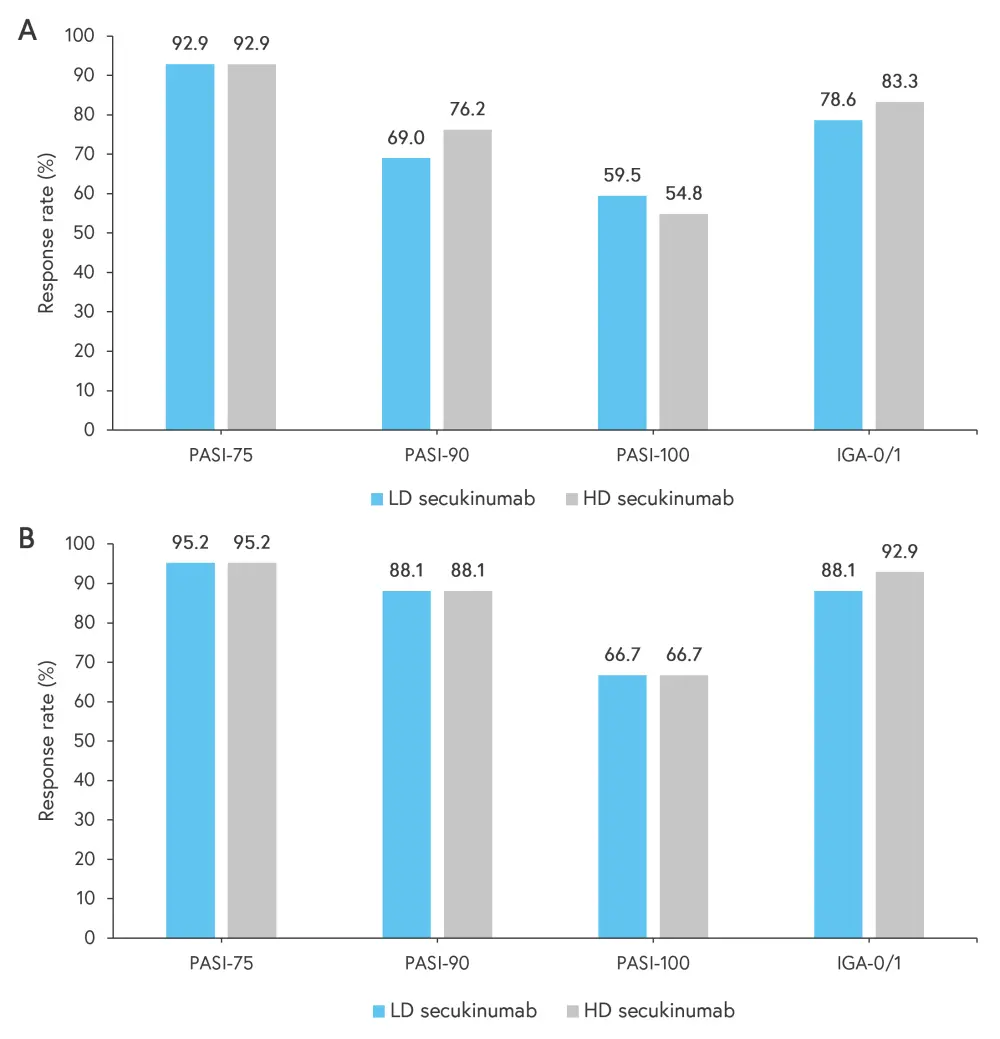
Outcomes at Weeks A 12 and B 24 across the LD and HD secukinumab treatment groups.
HD, high dose; IGA, Investigator Global Assessment; LD, low dose; PASI, Psoriasis Area and Severity Index.
*Data from Magnolo, et al.1
There were improvements in PASI, IGA-0/1, and Children’s Dermatology Quality of Life Index (CDQLI) scores from baseline at 12 weeks, and these improvements continued at 24 week (Table 3). One patient in the HD group discontinued treatment due to a lack of efficacy.
Table 3. PASI, IGA, and CDLQI score response rates at Weeks 12 and 24 for the LD and HD secukinumab treatment groups*
|
CDLQI, Children’s Dermatology Quality of Life Index; HD, high dose; IGA, Investigator Global Assessment; LD, low dose; PASI, Psoriasis Area and Severity Index. |
||
|
Score, % (unless otherwise stated) |
LD secukinumab |
HD secukinumab |
|---|---|---|
|
Improvement from mean baseline PASI at 12 weeks |
92.6 |
93.9 |
|
Improvement from mean baseline PASI at 24 weeks |
96.4 |
96.6 |
|
Mean baseline CDQLI, score (SD) |
10.6 (6.0) |
13.0 (7.0) |
|
Week 12 mean CDQLI, score |
2.1 |
2.2 |
|
Week 12 improvement from baseline CDQLI |
78.4 |
83.3 |
|
Week 24 mean CDQLI, score |
1.5 |
2.0 |
|
Week 24 improvement from baseline CDQLI |
88.1 |
92.9 |
|
Week 12 mean CDQLI decrease from baseline |
78.4 |
83.3 |
|
Week 24 mean CDQLI decrease from baseline |
86 |
80.6 |
|
Week 12 CDQLI of 0/1 |
50.0 |
61.9 |
|
Week 24 CDQLI of 0/1 |
70.7 |
60.5 |
Secukinumab was generally well tolerated, with a similar safety profile to that seen in the phase III trials performed in adults. The most common adverse events by system organ class were infections and infestations, with nasopharyngitis and acne the most common by preferred term (Table 4). Two patients in the HD group discontinued treatment due to adverse events and there were no treatment-related deaths. Of note, Candida infections were less frequent (1.2%) in the pediatric population compared with studies in adults (2.3–4.7%).3
Table 4. Adverse events*
|
AE, adverse event; HD, high dose; LD, low dose; TEAE, treatment-emergent adverse event. |
||
|
AE, % (unless otherwise stated) |
LD secukinumab |
HD secukinumab |
|---|---|---|
|
Any TEAE |
57.1 |
57.1 |
|
Most common AE by system organ class |
|
|
|
Infections and infestations |
35.7 |
42.9 |
|
Gastrointestinal disorders |
9.5 |
19.0 |
|
Skin/subcutaneous disorders |
9.5 |
9.5 |
|
Most common AE by preferred term |
|
|
|
Nasopharyngitis |
14.3 |
9.5 |
|
Acne |
7.1 |
2.4 |
|
Diarrhea |
0.0 |
7.1 |
|
Leukopenia |
4.8 |
2.4 |
|
Neutropenia |
4.8 |
2.4 |
|
Pyrexia |
7.1 |
0.0 |
|
Upper respiratory tract infection |
0.0 |
7.1 |
Conclusion
Overall, the 24 week analysis demonstrates that secukinumab is well tolerated in pediatric patients with plaque psoriasis and is efficacious at achieving a clinical response and improving quality of life in this population. The study is ongoing and aims to assess long-term efficacy and safety over a 4-year period. The main limitations of the trial are it’s open-label nature and the lack of a placebo or control group.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

