All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Safety and efficacy of bimekizumab for PsA up to 52 weeks: BE VITAL open-label extension
Psoriatic arthritis (PsA) can occur across multiple domains, with treatment aiming to achieve optimal outcomes in all affected domains. Tumor necrosis factor inhibitors (TNFi) are often used as a first-line treatment in patients with active PsA; however, there is a population of patients who do not yield an adequate response to TNFi.
Here, we summarize an article by Coates et al.1 published in RMD Open on the results up to Week 52 in the BE VITAL open-label extension (OLE), which followed on from the Phase III BE COMPLETE trial. BE VITAL investigated the long-term safety and efficacy of bimekizumab, an anti-interleukin 17 antibody. This analysis focuses on patients who do not yield an adequate response to TNFi.
Study design1
-
BE COMPLETE was a 16-week phase III placebo-controlled trial. Additional information about BE COMPLETE has previously been published on the Psoriasis and Psoriatic Arthritis Hub.
-
Patients who completed the BE COMPLETE trial entered the BE VITAL OLE, in which patients received bimekizumab 160 mg every 4 weeks for up to 140 weeks.
-
Included patients who had active PsA with a baseline tender joint count ≥3, swollen joint count ≥3, and ≥1 active psoriatic lesion and/or a history of psoriasis.
-
Intolerance or an inadequate response to one or two TNFi for the treatment of psoriatic disease was required.
Key findings1
-
A total of 377 patients (256 randomized to bimekizumab and 12 randomized to placebo) were included in the BE VITAL OLE.
-
Efficacy, as measured by American College of Rheumatology scores and Psoriasis Area and Severity Index, was improved compared with placebo at Week 16, and sustained to Week 52 in patients treated with bimekizumab (Figure 1).
-
In patients who switched to bimekizumab from placebo, rapid improvements in American College of Rheumatology scores occurred after 12 weeks of treatment and were observed up to Week 52.
-
To Week 52, the most common treatment-emergent adverse events in patients treated with bimekizumab were COVID-19 infection (7.2%), oral candidiasis (6.2%), nasopharyngitis (5.9%), and urinary tract infection (5.9%).
-
All Candida infections in bimekizumab-treated patients were of mild or moderate severity.
-
There was one sudden death and one cerebral hemorrhage during BE VITAL, which were unrelated to the study treatment.
Figure 1. Efficacy outcomes (ACR 50, PASI 90, and MDA) at A Week 16 and B Week 52*
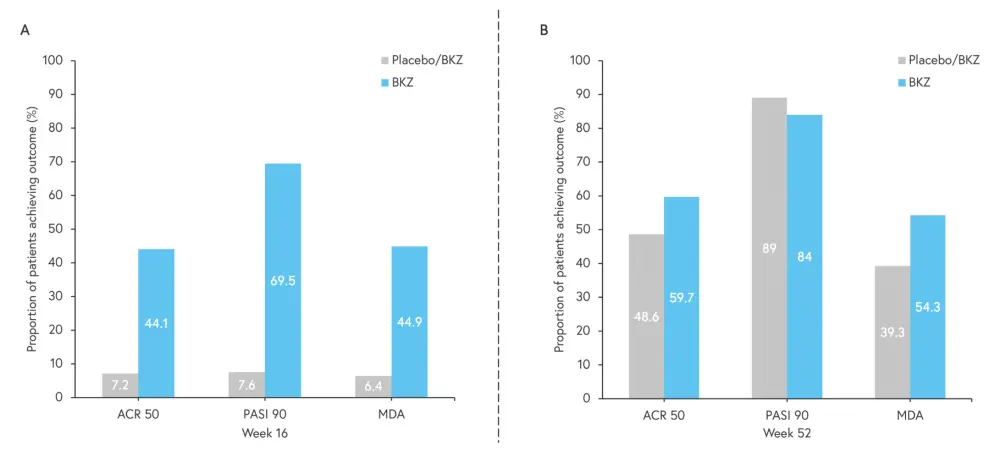
ACR 50, 50% improvement in American College of Rheumatology score; BKZ, bimekizumab; MDA, minimal disease activity; PASI 90, 90% improvement in Psoriasis Area and Severity Index.
*Data from Coates, et al.1
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?



