All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Bimekizumab for plaque psoriasis: 104-week results from BE BRIGHT
Do you know... In the BE BRIGHT trial, which TEAE was most common across all treatment groups between Week 56 and 104?
In the phase III BE SURE trial (NCT03412747), bimekizumab was shown to be superior to adalimumab in treating patients with moderate-to-severe plaque psoriasis at Week 16.1 The Psoriasis and Psoriatic Arthritis Hub has previously reported on the safety and efficacy of bimekizumab, including 1-year results from the BE SURE trial. Here, we discuss the updated results from BE SURE and 104-week outcomes from the ongoing open-label extension BE BRIGHT trial (NCT03598790).
Study design1
At Week 56, BE SURE patients were eligible to enter the BE BRIGHT extension to receive treatment for at least 144 weeks; if patients did not enrol, or discontinued treatment prior to Week 56, they received a safety follow-up visit at 20 weeks after their last treatment dose. The dosing schedule for patients entering BE BRIGHT was determined by attainment of a 90% improvement in Psoriasis Area and Severity Index (PASI 90) by the end of BE SURE (Week 56). The study design of BE SURE and BE BRIGHT are shown in Figure 1. At Week 80 (Week 24 of BE BRIGHT) the investigators could reduce the dosing schedule from every 4 weeks to every 8 weeks for patients receiving bimekizumab 320 mg, if PASI 90 was achieved.
Figure 1. Study design of BE SURE and BE BRIGHT*
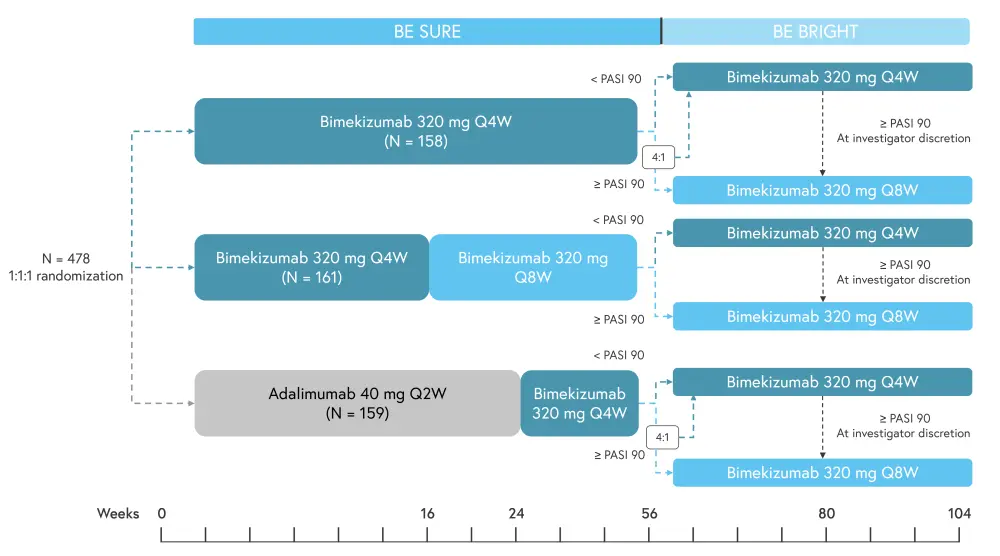
PASI 90, 90% improvement in the Psoriasis Area and Severity Index; Q4W, every 4 weeks; Q8W, every 8 weeks.
*Adapted from Thaci, et al.1
For patients enrolled in BE BRIGHT, final study assessment at Week 56 of BE SURE served as the baseline measurement. Baseline patient characteristics were similar across treatment groups.
Results1
Safety
The occurrence of treatment-emergent adverse events (TEAEs) by exposure-adjusted incidence rates of new cases per 100 patient-years for Weeks 56–104 of BE BRIGHT is shown in Figure 2. The most common TEAE was nasopharyngitis, followed by oral candidiasis and upper respiratory tract infection.
Figure 2. Incidence of TEAEs between Weeks 56–104*
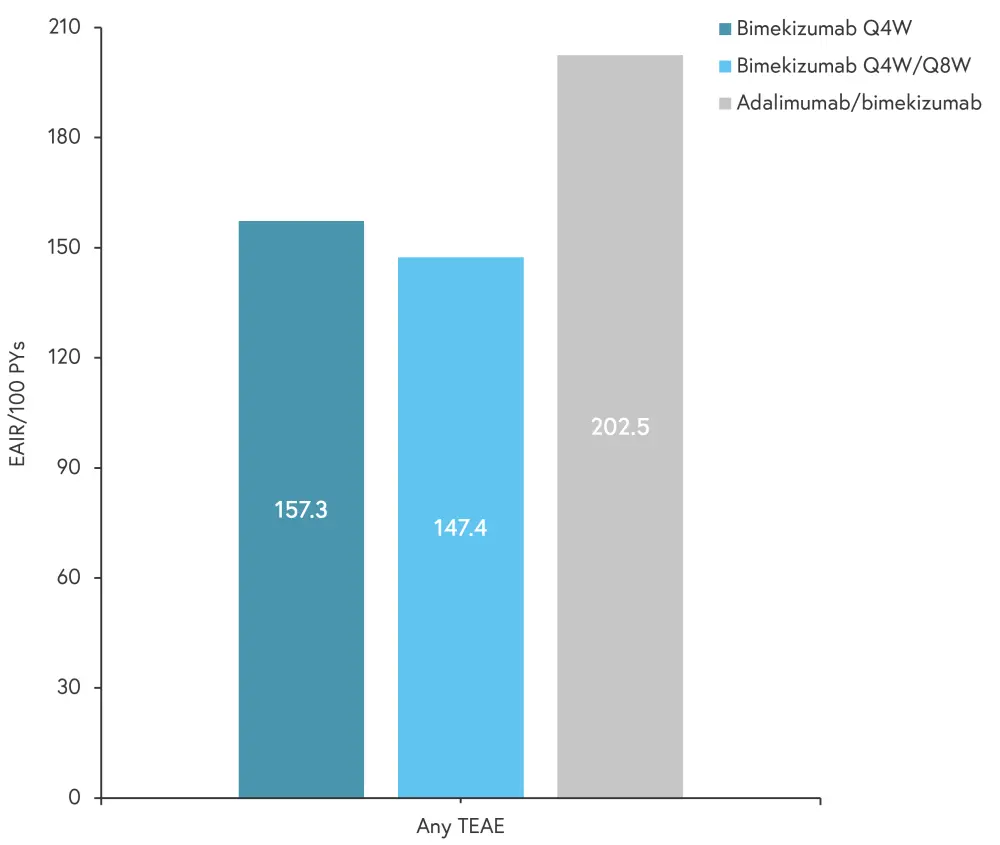
EAIR, exposure-adjusted incidence rate; PY, patient year; Q4W, every 4 weeks; Q8W, every 8 weeks; TEAE, treatment-emergent adverse event.
*Adapted from Thaci, et al.1
The rate of serious infections and malignancies was low across treatment groups. The rate of TEAEs decreased in patients who switched to bimekizumab from adalimumab, with the exception of opportunistic fungal infections and candidiasis which were highest between Week 24 and 56. There was only one death in the study, a 65-year-old patient receiving bimekizumab every 4 weeks, which was due to atherosclerosis; this was not considered treatment related when accounting for the patient’s multiple comorbidities.
The incidence of drug-related adverse events, discontinuations, and common TEAEs by exposure-adjusted incidence rates of new cases per 100 patient-years is shown in Figure 3.
Figure 3. Incidence of discontinuations, drug-related treatment-emergent adverse events, and most common TEAEs event between Week 56 and 104*
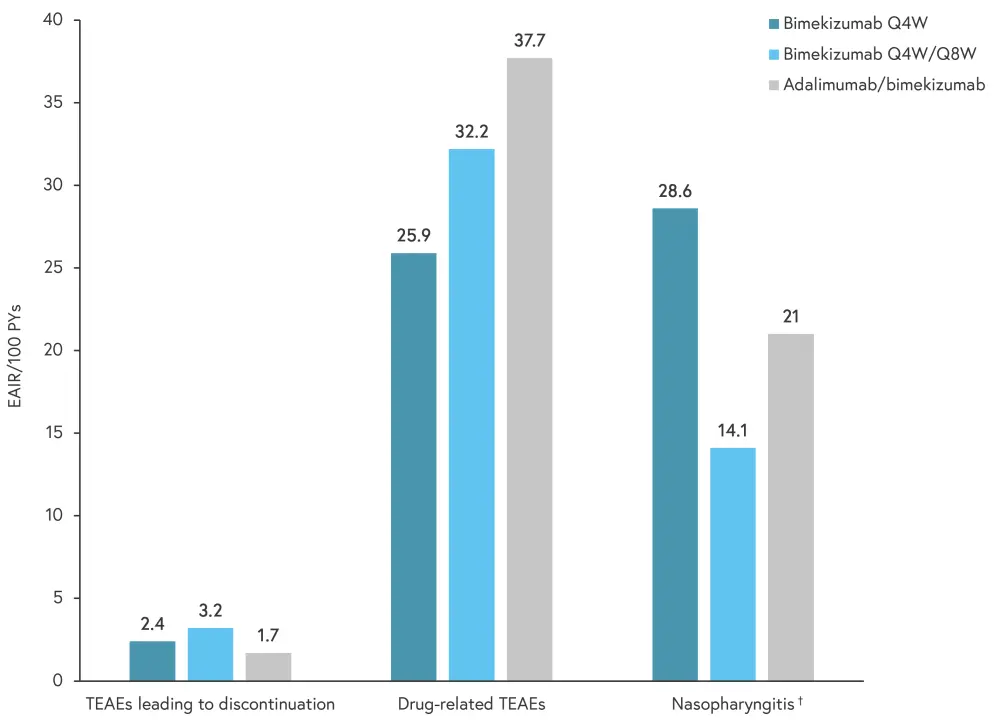
EAIRs, exposure-adjusted incidence rate; PY, patient year; Q4W, every 4 weeks; Q8W, every 8 weeks; TEAE, treatment-emergent adverse event.
*Adapted from Thaci, et al.1
†Most common TEAE.
Efficacy
Based on BE SURE treatment groups, outcomes were similar between study arms at Week 104 (Week 48 of BE BRIGHT; Figure 4).
Figure 4. Key efficacy outcomes at Week 104 by initial treatment group (modified nonresponder imputation)*
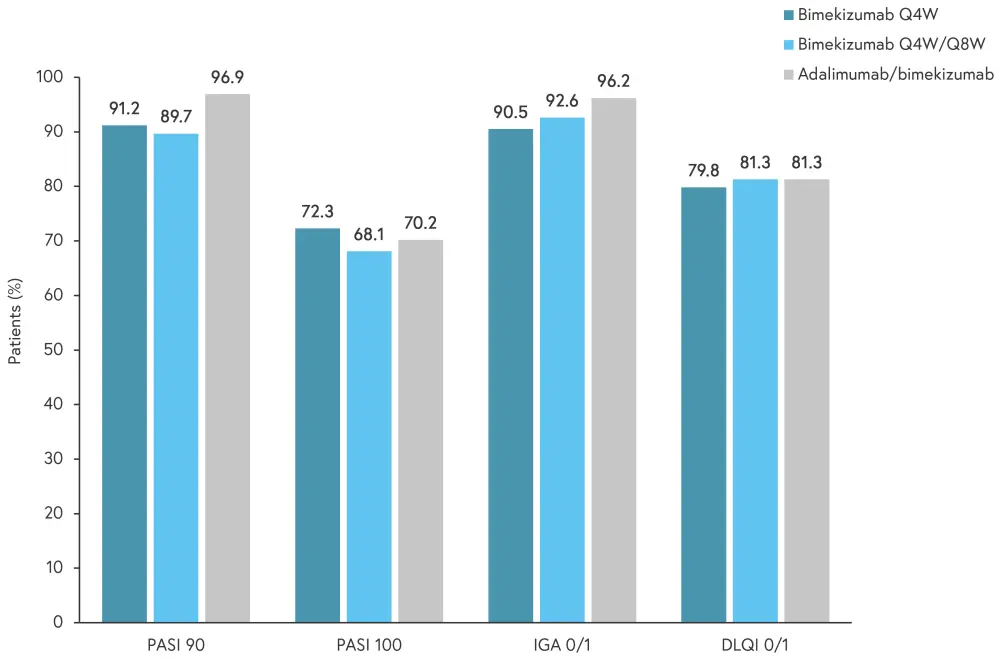
DLQI 0/1, Dermatology Life Quality Index score of 0 or 1; IGA 0/1, Investigator Global Assessment score of 0 or 1; PASI 90, % 90% improvement in Psoriasis Area and Severity index; PASI 100, 100% improvement in PASI; Q4W, every 4 weeks; Q8W, every 8 weeks.
*Adapted from Thaci, et al.1
Overall, PASI 90 results were similar to Week-16 of BE SURE and were maintained to Week 104. In patients who were originally randomized adalimumab during BE SURE, there was an increased response after treatment was switched to bimekizumab at Week 24; this increase was also durable to Week 104.
Conclusion
Among patients with plaque psoriasis, several did not achieve skin clearance or experience loss of efficacy over time. In both BE SURE and BE BRIGHT, outcomes demonstrated no additional safety concerns when switching from adalimumab to bimekizumab; these patients showed similar efficacy to patients who received bimekizumab for the full study duration. These findings suggest that responses were durable in patients treated with both bimekizumab every four or eight weeks up to Week 104, indicating that less frequent dosing may be efficacious for long-term maintenance treatment. Furthermore, the high rate of patients achieving a Dermatology Life Quality Index score of 0 or 1 suggests that bimekizumab may improve quality of life in patients with plaque psoriasis. There were no new safety signals observed in BE BRIGHT and bimekizumab was generally well tolerated. The authors noted several limitations of this study, including the difficulty of drawing conclusions from a clinical setting that apply to real-world outcomes; treatment survival with biologics is often higher in clinical research. However, these results demonstrate the durable efficacy of bimekizumab throughout the 2-year treatment period regardless of dosing regimen.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with psoriatic arthritis do you see per month?

