All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Approved and investigational JAK inhibitors for psoriatic arthritis
Pro-inflammatory pathways in the pathogenesis of psoriatic arthritis (PsA) have traditionally been managed by treatment with conventional synthetic (cs) disease-modifying antirheumatic drugs (DMARDs) and biologic (b) DMARDs.1 However, in some patients, the disease will not respond to these drugs or they may become intolerant over time.1 Therefore, the development of other treatments that can control inflammation associated with PsA is a priority.
In recent years, several Janus kinase (JAK) inhibitors have been approved for treatment of patients with active PsA. Here, we summarize the current and pipeline JAK inhibitors available for PsA.
JAK-STAT pathway2
The JAK-signal transducer and activator of transcription (STAT) pathway is shown in Figure 1.
Figure 1. The JAK-STAT pathway*
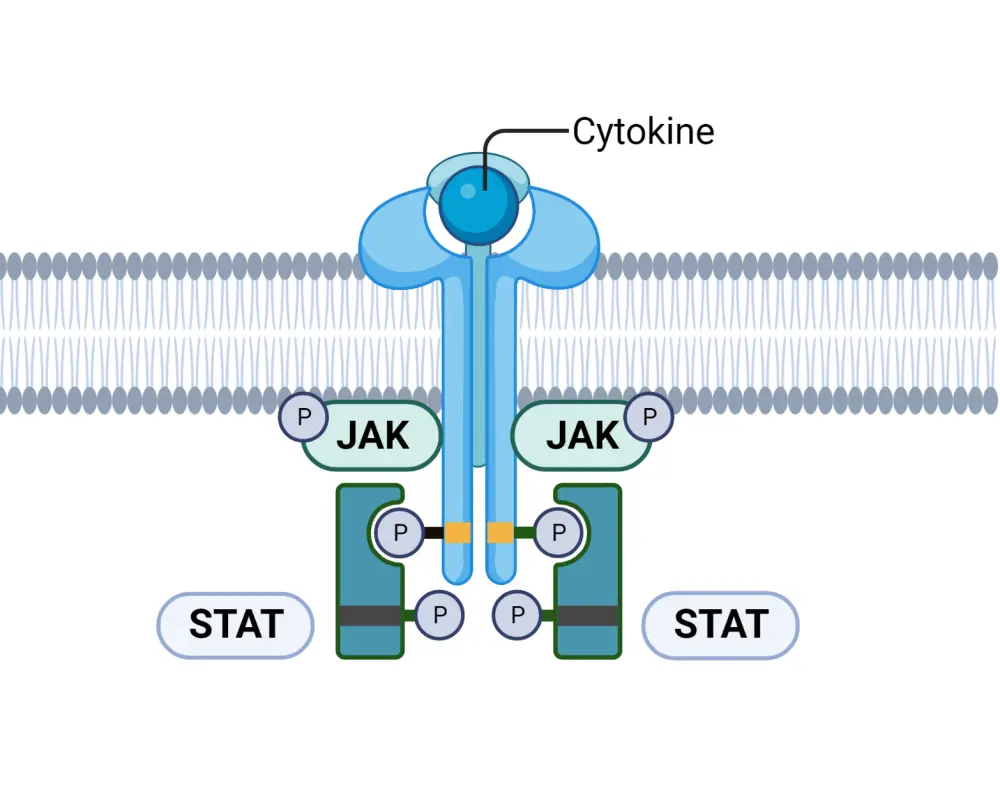
JAK, Janus kinase; STAT, signal transducer and activator of transcription.
*Created with BioRender.com
To summarize, pro-inflammatory cytokines, including interleukins (ILs) and interferons (IFNs), attach to the cytokine receptor on the plasma membrane. Binding causes the JAK molecules to transphosphorylase, which in turn phosphorylates tyrosine kinase; this leads to recruitment of STAT and the transcription of genes that code for pro-inflammatory cytokines, such as tumor necrosis factor α, IL1β, IL-6, IL-23, and IL-17. Use of JAK inhibitors can disrupt this pro-inflammatory pathway in patients with PsA.
Overview1
An outline of JAK inhibitors currently approved and under investigation for PsA is shown in Figure 2.
Figure 2. Overview of JAK inhibitors for PsA*
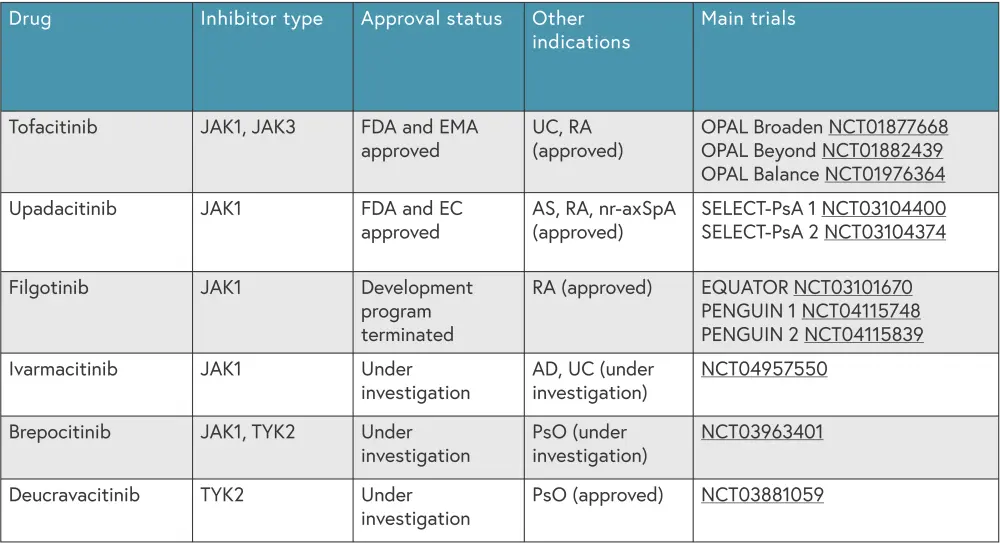
AD, atopic dermatitis; AS, ankylosing spondylosis; EC, European Commission; EMA, European Medicines Agency; FDA, Food and Drug Administration; JAK, Janus Kinase; nr-axSpA, non-radiographic axial spondyloarthritis; PsO, psoriasis; RA, rheumatoid arthritis; TYK2, tyrosine Kinase 2; UC, ulcerative colitis.
*Data from Caso, et al.1
Approved JAK inhibitors
Tofacitinib
Tofacitinib is an oral JAK1 and JAK3 inhibitor and minimally inhibits JAK2 and TYK2.1 Tofacitinib was approved by the U.S Food and Drug Administration (FDA) in 2017 and by the European Medicines Agency in 2018 for use in patients with active PsA. 3,4
The safety and efficacy of tofacitinib was evaluated in the phase III OPAL Broaden (NCT01877668) and OPAL Beyond (NCT01882439) trials, results of which have been partially reported on the Psoriasis and Psoriatic Arthritis Hub.1 In short, there was a significantly higher proportion of patients achieving a 20% improvement in American College of Rheumatology response (ACR20) when treated with tofacitinib compared with placebo at 3 months. Tofacitinib has also demonstrated long-term safety and efficacy over 30 weeks in OPAL Balance (NCT01976364). The main adverse events noted in the OPAL trials were nasopharyngitis, upper respiratory tract infection, headache, and gastrointestinal events.1
The European Alliance of Associations for Rheumatology (EULAR) recommend that tofacitinib as a second line therapy after failure with tumor necrosis factor inhibitors, or in patients with peripheral arthritis who did not have a response to treatment with a csDMARD or bDMARD.1
Upadacitinib
Upadacitinib, an oral JAK1 inhibitor which minimally inhibits JAK2,1 was approved by the FDA5 and European Commission6 in 2021 for use in patients with active PsA. The SELECT PsA 1 (NCT03104400) and SELECT PsA 2 (NCT03104374) trials evaluated the safety and efficacy of upadacitinib compared with adalimumab and placebo.1 Results from both trials demonstrated that upadacitinib was superior to placebo in improving PsA signs and patient-reported outcomes, with an acceptable safety profile. The 2021 Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) recommendations advise that upadacitinib can be used to treat multiple PsA domains.1
JAK1 inhibitors under investigation
Filgotinib
Filgotinib, a JAK1 inhibitor, was approved for use in Europe in 2020 for rheumatoid arthritis.7 The phase II EQUATOR trial (NCT03101670), evaluating filgotinib in patients with active PsA who were intolerant to one or more csDMARD, demonstrated that a higher proportion of filgotinib treated patients achieved ACR20 at Week 16 compared with placebo.1 The main adverse events observed were nasopharyngitis and headache, affecting 57% and 59% of filgotinib treated patients and placebo treated patients, respectively.
Two phase III trials of filgotinib for active PsA, PENGUIN 1 (NCT04115748) and PENGUIN 2 (NCT04115839) were paused and subsequently terminated after the FDA rejected a New Drug Application for filgotinib in rheumatoid arthritis. Therefore, filgotinib is no longer being developed for PsA at this time.1
Ivarmacitinib1
Ivarmacitinib is an oral JAK1 inhibitor currently being investigated for PsA. A phase III trial of ivarmacitinib (NCT04957550) is currently enrolling patients with active PsA, with an estimated completion date in 2024. The trial will compare two doses of ivarmacitinib to placebo over 48 weeks.
TYK2 inhibitors under investigation1
Brepocitinib
Brepocitinib, an oral TYK2 and JAK1 inhibitor, has previously been described as safe and effective in patients with plaque psoriasis. A phase IIb trial (NCT03963401) recruited patients with PsA who were intolerant to non-steroidal anti-inflammatory drugs/DMARDs and compared brepocitinib (doses of 10 mg, 30 mg, and 60 mg) to placebo over 52 weeks. Significantly more patients treated with 30 mg or 60 mg brepocitinib achieved ACR20 at Week 16 compared with placebo (67% and 75% vs 43%, respectively). There were no major cardiovascular events, serious infections, or deaths over the 52 weeks; this suggests that brepocitinib may be a viable option for patients with active PsA.
Deucravacitinib
Deucravacitinib is an oral TYK2 inhibitor that is already FDA and European Commission approved for adults with moderate-to-severe plaque psoriasis.8 A phase II trial (NCT03881059) evaluated deucravacitinib (6 mg and 12 mg) versus placebo in patients with PsA, with a higher proportion of patients treated with deucravacitinib achieved ACR20 at Week 16 compared with placebo (52.9% and 62.7% vs 31.8%, respectively). Deucravacitinib was also found to have similar efficacy in patients who had received previous treatment with a csDMARD and those who had not. The safety profile at Week 52 was found to be similar to previous phase III trials in patients with plaque psoriasis.
Figure 3 presents a comparison of patients achieving ACR20 when treated with investigational TYK2 inhibitors.
Figure 3. Percentage of patients achieving ACR20 when treated with A brepocitinib and B deucravacitinib*
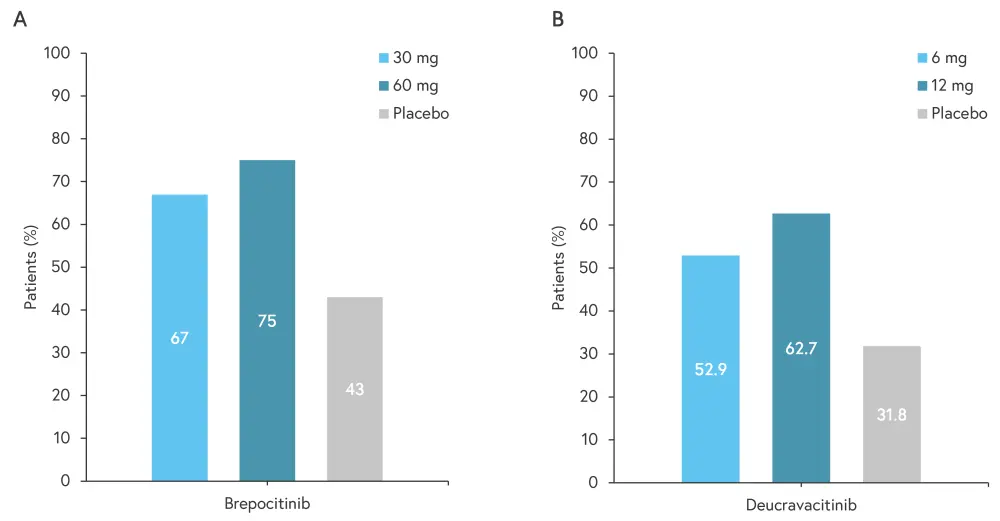
ACR20, 20% improvement in American College of Rheumatology response.
*Adapted from Caso, et al.1
Conclusion
The fast and effective treatment of PsA is essential to improve patient quality of life. With the development of new treatments targeting the JAK-STAT pathway, patients with PsA have further treatment options, especially those who are intolerant to DMARDs.1 JAK inhibitors have the benefit of oral administration which can improve treatment compliance, with two currently approved JAK inhibitors, tofacitinib and upadacitinib, shown to be effective and safe for use in patients with PsA.1 The preliminary results for other JAK1 and TYK2 inhibitors are promising and more inhibitors are likely to be approved in the near future.1
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

