All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
A post hoc analysis of DAPSA and MDA targets for measuring disease activity in patients with PsA
Do you know... Which of the following measures does not form part of DAPSA scores?
Clinical remission, or achievement of low disease activity (LDA), is one of the key objectives of psoriatic arthritis (PsA) treatment.1 However, measurement of disease activity remains a challenge, as there is no single tool covering all domains of PsA. It is unclear whether tools that measure a single domain versus a multi-domain are more effective for the measurement of PsA disease activity.
Tofacitinib, a Janus kinase inhibitor, has shown promising efficacy in patients with PsA. The Psoriasis and Psoriatic Arthritis Hub has previously reported the baseline cardiovascular risk and incidence of major cardiovascular adverse events or malignancies in patients with PsA and psoriasis receiving tofacitinib. Here, we summarize a post hoc analysis of the phase III, randomized, placebo-controlled trials of tofacitinib in patients with PsA recently published by Schneeberger et al.1 in Seminars in Arthritis and Rheumatism. This post hoc analysis pooled data from the OPAL BROADEN (NCT01877668) and OPAL BEYOND (NCT01882439) trials and compared the proportion of patients achieving disease activity index for psoriatic arthritis (DAPSA) remission (REM) and LDA with achievement of very low disease activity (VLDA) and minimal disease activity (MDA).1
Study design
Both trials were double-blind, placebo-controlled, and enrolled patients with active PsA. OPAL BROADEN was a 12-month trial and OPAL BEYOND was a 6-month trial. The current post hoc analysis included data from patients who received tofacitinib 5 mg (n = 237) and tofacitinib 10 mg (n = 236) twice daily. The DAPSA and MDA responses were evaluated at baseline and Months 1, 3, and 6 in both trials. In OPAL BROADEN, these measures were also evaluated at Months 9 and 12. The DAPSA measures were determined by adding together tender joint count in 68 joints (TJC68), swollen joint count in 66 joints (SWJ66), patient global assessment (PtGA) of PsA activity (visual analog scale [VAS] in cm), Patient Pain Assessment (VAS in cm), and Composite Psoriatic Disease Activity Index (mg/dL).
For VLDA and MDA, the following criteria were measured:
- TJC68 ≤1
- SJC66 ≤1
- Psoriasis Area and Severity Index (PASI) ≤1 or body surface area ≤3%
- PtGA of PsA activity (VAS in cm) ≤20 mm
- Patient pain assessment (VAS in cm) ≤15 mm
- Health Assessment Questionnaire-Disability Index (HAQ-DI) ≤0.5
- Tender entheseal points ≤1
MDA was defined as having five or six of the criteria and VLDA as having all seven of the criteria above. HAQ-DI and Short Form-36 Health Survey (SF-36) Physical Component Summary (PCS) scores were also measured at baseline and Month 6.
Results
Patient characteristics and responses at 3 months
Baseline patient characteristics were generally similar across the treatment arms. At Month 3, female patients or patients with increased disease activity at baseline were less likely to achieve all targets (DAPSA-REM/DAPSA-LDA and VLDA/MDA). In addition, older patients at baseline were less likely to achieve the DAPSA targets. Patients who achieved VLDA at Month 3 generally had lower PASI scores; however, the PASI score at baseline was not found to be significant in VLDA/MDA outcomes at Month 3.
Achievement of DAPSA-REM/DAPSA-LDA and VLDA/MDA targets
From Months 1–6, the percentage of patients achieving the targets increased for all measures, as shown in Figure 1. At Months 1, 3, and 6, around half of the patients who achieved DAPSA-REM did not achieve VLDA and around two-thirds of patients who achieved DAPSA-LDA did not achieve MDA. Only 11 patients achieved VLDA or MDA targets without also achieving DAPSA-REM or DAPSA-LDA.
Figure 1. Percentage of patients achieving the target at Months 1,3, and 6 for A DAPSA-REM versus VLDA, and B DAPSA-LDA versus MDA*
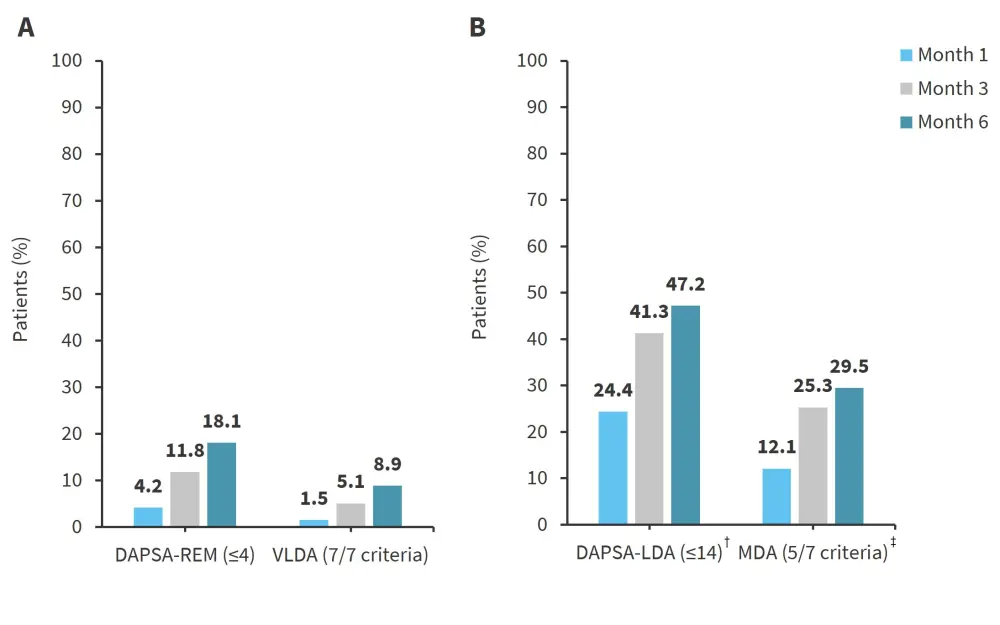
DAPSA-LDA, disease activity index for psoriatic arthritis low disease activity; DAPSA-REM, DAPSA remission; MDA, minimal disease activity; VLDA, very low disease activity.
*Adapted from Schneeberger, et al.1
†DAPSA-REM (≤4) or DAPSA-LDA (>4 to ≤14).
‡MDA (5/7), by definition, includes VLDA (7/7).
The mean PASI response in patients who achieved both DAPSA-REM and VLDA at Month 6 was 0.4, compared with 3.5 in patients who achieved DAPSA-REM without VLDA. There was a smaller difference in mean PASI score between patients who achieved DAPSA-LDA with and without MDA (3 vs 4.2). The mean PtGA of PsA activity score also varied in patients who achieved DAPSA targets with and without MDA targets, with a mean score of 4.6 versus 6.7 in patients achieving DAPSA-REM with and without VLDA, and a mean score of 12.8 versus 26.7 for patients achieving DAPSA-LDA with and without MDA, respectively.
Association between quality-of-life targets and DAPSA/MDA targets
Patients who met the DAPSA and MDA targets at Month 6 had greater improvements in HAQ-DI and SF-36 PCS scores (Figure 2) from baseline scores compared with patients who did not meet these targets. The mean decrease in HAQ-DI was similar in patients who achieved DAPSA-REM and VLDA, even though DAPSA does not use a physical function measurement, and MDA includes the HAQ-DI score.
Figure 2. Mean change from baseline in A HAQ-DI and B SF-36 PCS score*
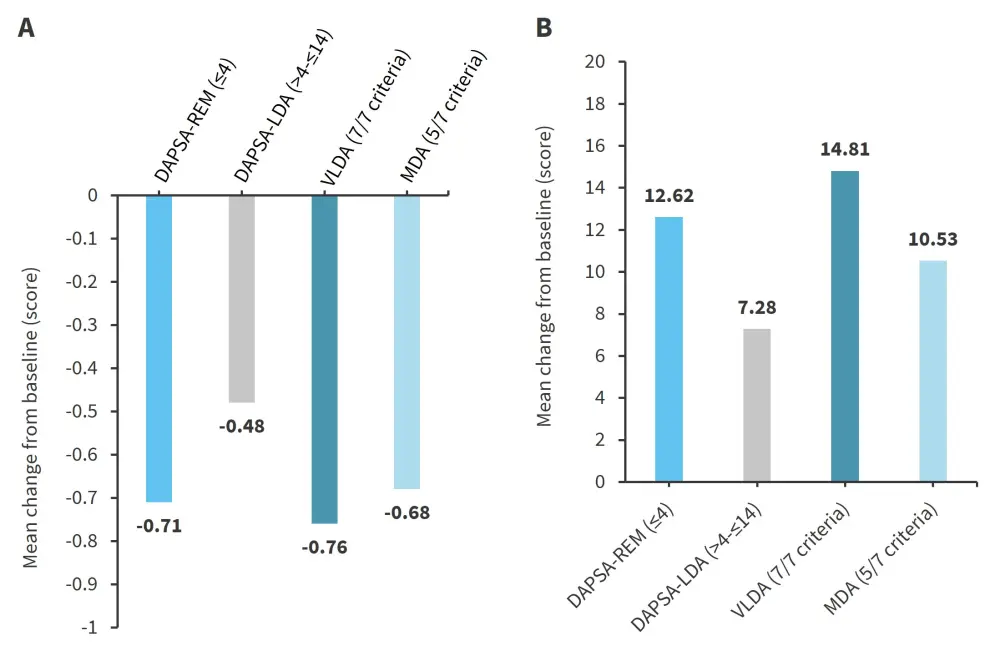
DAPSA-LDA, disease activity index for psoriatic arthritis low disease activity; DAPSA-REM, DAPSA remission; MDA, minimal disease activity; VLDA, very low disease activity.
*Adapted from Schneeberger, et al.1
Conclusion
This post hoc analysis has demonstrated that MDA targets can often be more difficult to achieve compared with DAPSA targets in patients with PsA treated with tofacitinib. There was a greater mean disease duration in patients who achieved DAPSA-REM without VLDA compared with those achieving VLDA. Disease duration is associated with greater joint damage and disability and therefore, patients with a longer disease duration are unlikely to meet the VLDA criteria despite meeting DAPSA criteria. This suggests that including quality-of-life measures such as HAQ-DI in disease activity measures such as VLDA may give an impression of poor outcomes in patients.
It is important to note that MDA/VLDA are categorical measures of disease activity compared with DAPSA on a continuous scale, meaning DAPSA measures may have greater use in trials where patients are measured continuously throughout treatment. Using DAPSA, patients can score differently in one disease component compared with another, with all individual components considered in the final outcome. In MDA/VLDA, patients must fulfill five to seven of the pre-specified criteria to achieve MDA or VLDA, suggesting it is a less specific method of disease activity measurement than DAPSA.
This analysis is limited as the OPAL trials were not originally designed to compare outcome measures in this way, and the use of only two studies resulted in a relatively small patient sample size. Therefore, additional larger studies designed for the purpose of comparing disease activity outcome. measures would be useful. Research is ongoing to determine how to produce an optimal disease activity composite measurement tool for use in patients with PsA, which can measure both skin and musculoskeletal domains of PsA.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?


