All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Baseline cardiovascular risk and incidence of major cardiovascular adverse events or malignancies in patients with PsA and PsO receiving tofacitinib
During the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, Lars Erik Kristensen presented the results of a study investigating the impact of comorbidities, such as metabolic syndrome (MetS) and cardiovascular disease (CVD), present in patients with psoriasis (PsO) and psoriatic arthritis (PsA).1 We are pleased to provide a summary of this presentation here.
In patients with PsO and PsA, MetS and CVD are described as common comorbidities. An increased incidence of malignancies may be associated with the increased risk of CVD in these patients due to a chronic inflammatory disease state. This prompted the team to explore the association between patients’ baseline cardiovascular risk and the incidence rate of major cardiovascular adverse events and development of malignancies following treatment with the Janus kinase inhibitor, tofacitinib.
Study design
The data presented in this study were gathered from a post hoc analysis of ten clinical trials that enrolled patients being given at least one dose of tofacitinib (5 or 10 mg twice daily), including the following:
- PsA studies
- Phase III: OPAL Broaden (NCT01877668) and OPAL Beyond (NCT01882439)
- Long-term extension: OPAL Balance (NCT01976364)
- PsO studies
- Phase II: NCT00678210 and NCT01710046
- Phase III: OPT Compare (NCT01241591), OPT Retreatment (NCT01186744), OPT Pivotal 1 (NCT01276639), and OPT Pivotal 2 (NCT01309737)
- Long term extension: OPT extend (NCT01163253)
The key study outcome was the incidence rate (patient events/100 patient-years) of myocardial infarctions, stroke, and cardiovascular death (collectively known as MACE) and malignancies (excluding non-melanoma skin cancer).
Patients were stratified according to:
- A history of coronary artery disease (CAD; defined as having ≥1 myocardial infarction, coronary heart disease, coronary artery, or stable angina pectoris event)
- Baseline 10-year risk of atherosclerotic CVD assessed using the ASCVD-PCE calculator2 (in patients without a history of CAD)
- Baseline MetS (defined as ≥3 of the following: hypertension, raised triglycerides, reduced high density lipoprotein cholesterol, high waist circumference, or high fasting glucose levels)
Follow-up time was recorded from the day of the first event to 28 days after the last dose of the study drug.
Results
The patient characteristics with respect to tofacitinib and history of CAD and MetS are shown in Table 1. The total tofacinitib exposure in patient years for patients with PsO was almost 9,000, whereas for patients with PsA, it was ~2,000. There is a trend towards increased risk of CAD and baseline MetS in patients transitioning from PsO to PsA.
Table 1. Patients with PsO and PsA characteristics*
|
CAD, coronary artery disease; MetS, metabolic syndrome; PsA, psoriatic arthritis; PsO, psoriasis. |
||
|
|
PsA (n = 783) |
PsO (n = 3,663) |
|---|---|---|
|
Total tofacitinib exposure, patient years |
2,038 |
8,950 |
|
Median duration of exposure, years (range) |
3.0 (0−4.8) |
2.4 (0−5.7) |
|
History of CAD, % |
5.0 |
2.5 |
|
Baseline MetS, % |
40.9 |
32.7 |
In patients with PsO or PsA who had no history of CAD, >20% presented with either a high or intermediate risk of baseline 10-year atherosclerotic CVD risk (Figure 1).
Figure 1. Baseline 10-year risk of atherosclerotic CVD in patients without a history of CAD*
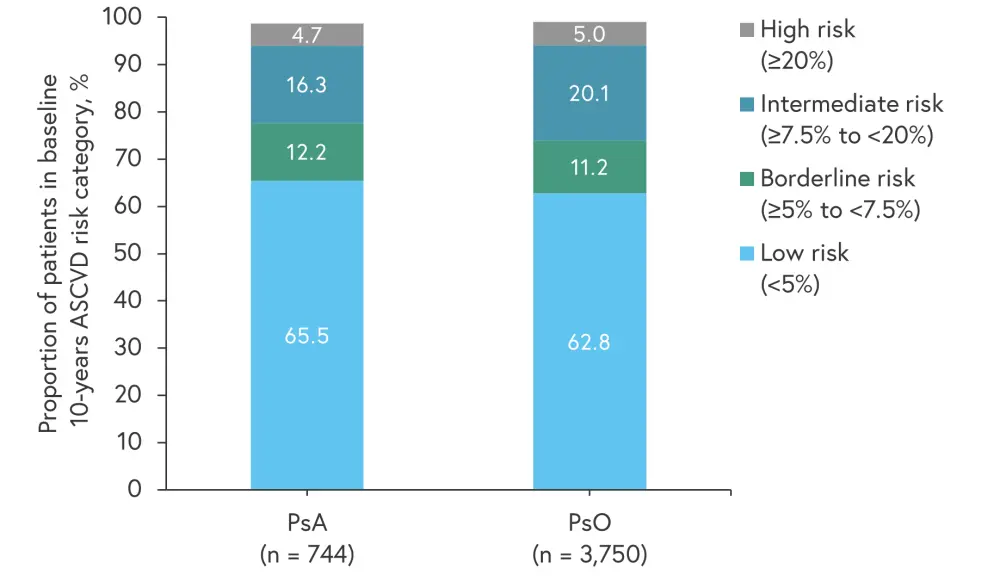
ASCVD, atherosclerotic cardiovascular disease; CAD, coronary artery disease; CVD, cardiovascular disease; PsA, psoriatic arthritis; PsO, psoriasis.
*Adapted from Lars Erik Kristensen.1
Baseline demographics are shown in Tables 2 and 3 for patients with PsA and Pso, respectively. Across all risk groups, the percentage of female patients with PsA was higher than males, while in the PsO group the opposite trend was seen. Baseline hypertension was higher in patients with PsA compared with PsO.
Table 2. Baseline demographics according to risk category for patients with PsA*
|
HLP, hyperlipidemia; HTN, hypertension; HxCAD, history of coronary artery disease; MetS, metabolic syndrome; PsA, psoriatic arthritis. |
|||||
|
Variable, % |
Low (<5%) |
Borderline (≥5 to <7.5%) |
Intermediate (≥7.5 to <20%) |
High (≥20%) |
HxCAD |
|---|---|---|---|---|---|
|
MetS |
27 |
56 |
63 |
89 |
67 |
|
Age ≥50/≥60 years |
30/5 |
79/26 |
90/49 |
100/86 |
80/46 |
|
Male |
36 |
44 |
67 |
74 |
64 |
|
Current/past smoker |
14/18 |
28/21 |
29/24 |
17/37 |
15/23 |
|
History of diabetes |
7 |
14 |
22 |
60 |
28 |
|
History of HTN |
23 |
51 |
66 |
89 |
82 |
|
History of HLP |
12 |
35 |
32 |
46 |
54 |
|
Baseline statins |
5 |
22 |
22 |
43 |
36 |
|
Baseline antiplatelet agents |
1 |
8 |
9 |
23 |
54 |
Table 3. Baseline demographics according to risk category for patients with PsO*
|
HLP, hyperlipidemia; HTN, hypertension; HxCAD, history of coronary artery disease; MetS, metabolic syndrome; PsO, psoriasis. |
|||||
|
Variable, % |
Low (<5%) |
Borderline (≥5 to <7.5%) |
Intermediate (≥7.5 to <20%) (n = 716) |
High (≥20%) |
HxCAD |
|---|---|---|---|---|---|
|
MetS |
22 |
42 |
48 |
74 |
56 |
|
Age ≥50/≥60 years |
16/1 |
58/12 |
79/32 |
93/68 |
75/44 |
|
Male |
64 |
70 |
84 |
84 |
76 |
|
Current/past smoker |
32/22 |
48/23 |
49/27 |
52/27 |
24/44 |
|
History of diabetes |
5 |
14 |
27 |
63 |
38 |
|
History of HTN |
12 |
30 |
36 |
65 |
58 |
|
History of HLP |
14 |
29 |
38 |
52 |
62 |
|
Baseline statins |
7 |
16 |
22 |
37 |
46 |
|
Baseline antiplatelet agents |
3 |
7 |
11 |
28 |
51 |
Incidence ratios
The MACE incidence rate (IR) for patients with PsA was found to be highest in patients with a history of CAD and with high baseline 10-year risk for atherosclerotic CVD. While there were low numbers of malignancies recorded in this study, a trend was seen of high IRs of malignancy being associated with patients with PsA in the intermediate and high baseline atherosclerotic CVD risk category.
In patients with PsO, the same pattern was observed in terms of highest association of MACE IR in patients with a history of CAD and high baseline 10-year atherosclerotic CVD risk. Concerning the IR of malignancies, results from patients with PsO included a larger number of total events recorded compared to patients with PsA and thus showed a clearer dose response. Patients in the high baseline 10-year atherosclerotic CVD risk category demonstrated the highest IR of malignancies followed by the intermediate risk group.
Limitations
The study was an exposure study and did not include a comparison arm. A low number of patients was included in certain CV risk subgroups. In the PsA cohort in particular, the total patient years for tofacitinib exposure was low. MetS was not assessed specifically at baseline but was analyzed post hoc. As this post hoc analysis included trials with different study design and diseases of different nature, i.e., PsO versus PsA, comparison of results was limited.
Conclusion
Patients with PsO and PsA that received tofacitinib and had an elevated baseline risk for CVD and MetS demonstrated a possible association with increased IRs for MACE and malignancies. Despite the limitations of this study, the presented results highlighted the importance of examining not only a patient’s skin and joint symptoms but also any comorbidities such as baseline CVD and MetS. Monitoring these patients for the development of cardiovascular or malignant sequalae will also be necessary and require multidisciplinary cooperation.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

