All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Upadacitinib vs adalimumab or placebo in patients with an inadequate response to non-biological therapy: Week 56 results from the SELECT-PsA 1 study
Do you know... SELECT-PsA 1 is a phase III trial evaluating upadacitinib for the treatment of patients with PsA who have had an inadequate response or are intolerant to 1 or more non-biological DMARD. Which treatment group had the highest percentage of patients achieving ACR70 at Week 56?
Psoriatic arthritis (PsA) is an inflammatory disease that can present with multiple clinical manifestations, including psoriasis, peripheral arthritis, dactylitis, enthesitis, spondylitis, quality of life, physical function, pain, and fatigue.1 Treatment strategies focus on maximizing patient outcomes by controlling inflammation and preventing irreversible joint damage and disability. Currently, treatment options for PsA include several disease-modifying antirheumatic drugs (DMARDs), yet <1/3 of patients with PsA achieve minimal disease activity (MDA) in most placebo-controlled trials so new treatment options are needed.1
Upadacitinib, an oral, reversible, Janus associated kinase (JAK) inhibitor, is approved in Europe for the treatment of rheumatoid arthritis, PsA, and ankylosing spondylitis.2 The SELECT-PsA 1 trial (NCT03104400) is investigating the effect of upadacitinib compared with adalimumab (standard of care treatment) or placebo in adult patients with active PsA and an inadequate response or intolerance to ≥1 non-biological DMARD. Results of the 56-week efficacy and safety analysis were published by Iain McInnes et al.1 in the journal Rheumatic & Musculoskeletal Diseases Open. A summary of these results is provided below.
Study design and patient characteristics
This was a phase III, randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of upadacitinib (15 mg or 30 mg once daily) compared with adalimumab (40 mg every other week) or placebo in 1,705 adult patients (≥18 years of age) with active PsA and inadequate response or intolerance to ≥1 non-biological DMARD. The study design can be seen in Figure 1.
Figure 1. Study design*
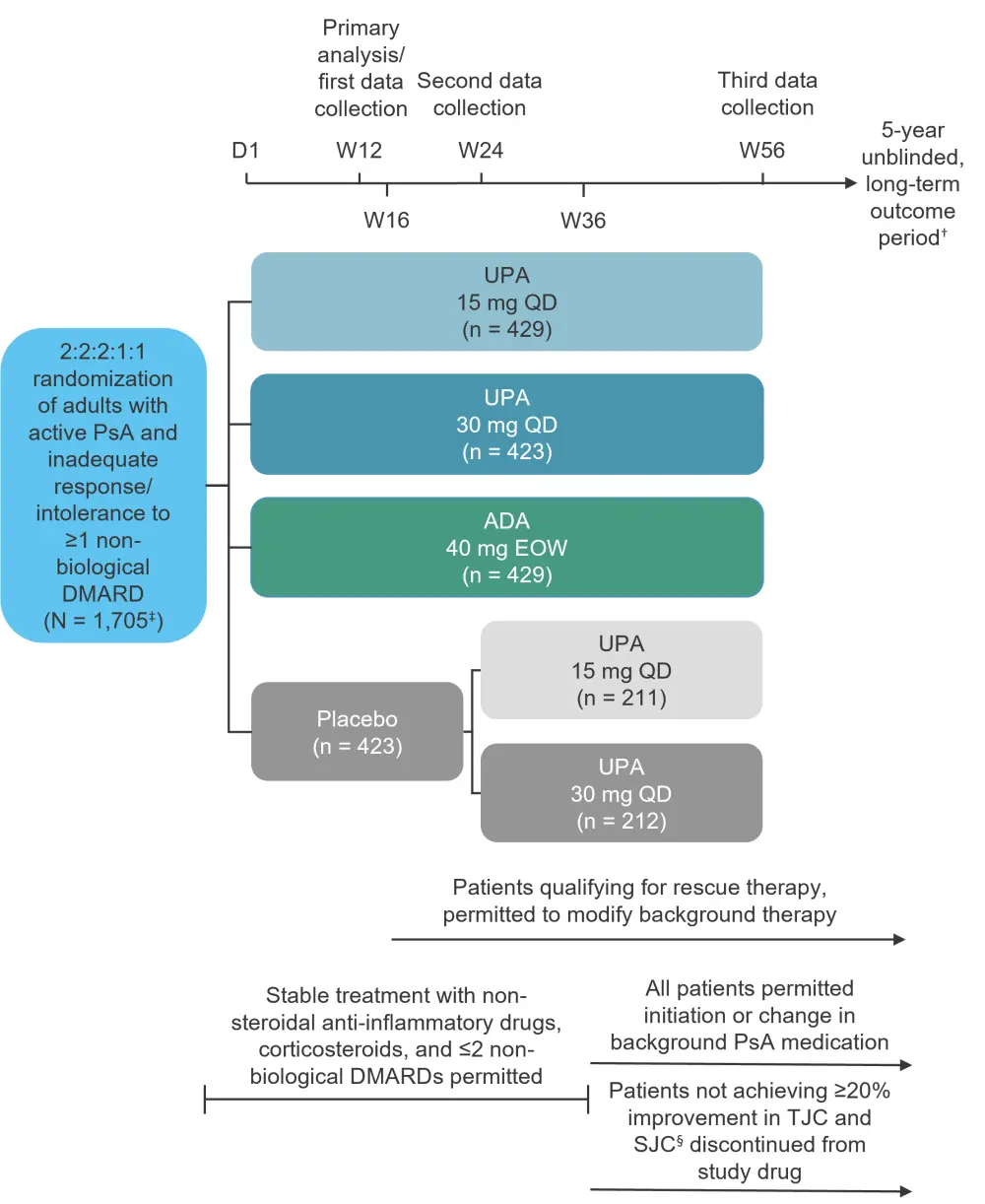
ADA, adalimumab; D, day; DMARD, disease-modifying antirheumatic drug; EOW, every other week; PsA, psoriatic arthritis; QD, once daily; SJC, swollen joint count; TCJ, tender joint count; UPA, upadacitinib; W, week.
*Data from McInnes et al.1
†Data from ClinicalTrials.gov.3
‡One patient in the UPA 15 mg cohort did not receive study drug.
§Compared with baseline at two consecutive visits.
Baseline characteristics were well balanced across all cohorts (Table 1).
Table 1. Baseline characteristics*
|
ADA, adalimumab; BSA, body surface area; DMARD, disease-modifying anti-rheumatic drug; EOW, every other week; HAQ-DI, Health Assessment Questionnaire - Disability Index; hs-CRP, high-sensitivity C-reactive protein; LDI, Leeds Dactylitis Index; LEI, Leeds Enthesitis Index; MTX, methotrexate; NB, non-biologic; NRS, numeric rating scale; NSAID, nonsteroidal anti-inflammatory drug; PASI, psoriasis area severity index; PsA, psoriatic arthritis; QD, once daily; SD, standard deviation; sIGA, Static Investigator Global Assessment of Psoriasis; SJC66, swollen joint count of 66 joints; TJC68, tender joint count of 68 joints; ULN, upper limit normal; UPA, upadacitinib. |
||||
|
Characteristic, % (unless otherwise stated) |
Placebo |
UPA |
UPA |
ADA |
|---|---|---|---|---|
|
Mean age ± SD, years |
50.4 ± 12.2 |
51.6 ± 12.2 |
49.9 ± 12.4 |
51.4 ± 12.0 |
|
Female |
49.9 |
55.5 |
55.8 |
51.7 |
|
Ethnicity, white† |
89.1 |
90.0 |
89.1 |
87.4 |
|
Mean time since PsA diagnosis ± SD, years |
6.2 ± 7.0 |
6.2 ± 7.4 |
5.9 ± 6.4 |
5.9 ± 7.1 |
|
Monotherapy |
18.0 |
17.7 |
18.2 |
19.1 |
|
Any NB DMARD at baseline‡ |
82.0 |
82.3 |
81.8 |
80.9 |
|
MTX alone |
63.1 |
65 |
63.4 |
62.9 |
|
MTX + another NB DMARD |
6.1 |
4.7 |
6.4 |
3.7 |
|
NB DMARD other than MTX |
12.8 |
12.6 |
12.1 |
14.2 |
|
Steroid use at baseline |
16.5 |
17.0 |
16.8 |
16.8 |
|
Mean TJC68 ± SD |
20.0 ± 14.3 |
20.4 ± 14.7 |
19.4 ± 13.3 |
20.1 ± 13.8 |
|
Mean SJC66 ± SD |
11.0 ± 8.2 |
11.6 ± 9.3 |
10.6 ± 7.1 |
11.6 ± 8.8 |
|
hs-CRP >ULN§ |
76.6 |
75.5 |
76.6 |
71.8 |
|
Mean HAQ-DI ± SD |
1.1 ± 0.6 |
1.2 ± 0.7 |
1.1 ± 0.6 |
1.1 ± 0.6 |
|
Mean patient’s assessment of pain (NRS 0–10) ± SD |
6.1 ± 2.1 |
6.2 ± 2.1 |
5.9 ± 2.1 |
6.0 ± 2.1 |
|
BSA-psoriasis ≥3% |
49.9 |
49.9 |
49.6 |
49.2 |
|
Mean PASI for baseline BSA-psoriasis ≥3% ± SD |
11.2 ± 11.4 |
9. 8 ± 10.0 |
9.5 ± 8.8 |
9.4 ± 8.5 |
|
BSA-psoriasis ≥0%, |
94.6 |
91.8 |
93.9 |
92.5 |
|
sIGA of psoriasis score |
|
|
|
|
|
0 |
5.7 |
7.9 |
5.0 |
7.9 |
|
1 |
20.3 |
17.0 |
18.4 |
15.2 |
|
2 |
39.5 |
39.6 |
40.9 |
42.2 |
|
3 |
28.1 |
31.0 |
30.3 |
30.8 |
|
4 |
6.4 |
4.4 |
5.4 |
4.0 |
|
Presence of enthesitis |
57.0 |
62.9 |
63.1 |
61.8 |
|
Presence of dactylitis |
29.8 |
31.7 |
30.0 |
29.6 |
Results
In total, 83.2% of the patients enrolled in the trial completed 56 weeks of treatment. The most common reasons for discontinuation were withdrawal by the patient and lack of efficacy.
Efficacy
The proportions of patients achieving ≥20%/50%/70% improvement in American College of Rheumatology response criteria (ACR20/50/70) response were maintained from Week 24 to Week 56 across all treatment groups. A greater proportion of patients originally randomized to upadacitinib achieved ACR20/50/70 compared with those randomized to adalimumab at Week 56 (non-responder imputation analysis: nominal p ≤ 0.05 for 15 mg upadacitinib vs adalimumab for ACR50/70; nominal p ≤ 0.05 for 30 mg upadacitinib vs adalimumab for ACR20/50/70). ACR70 responses can be seen in Figure 2.
Figure 2. Percentage of patients achieving ACR70*
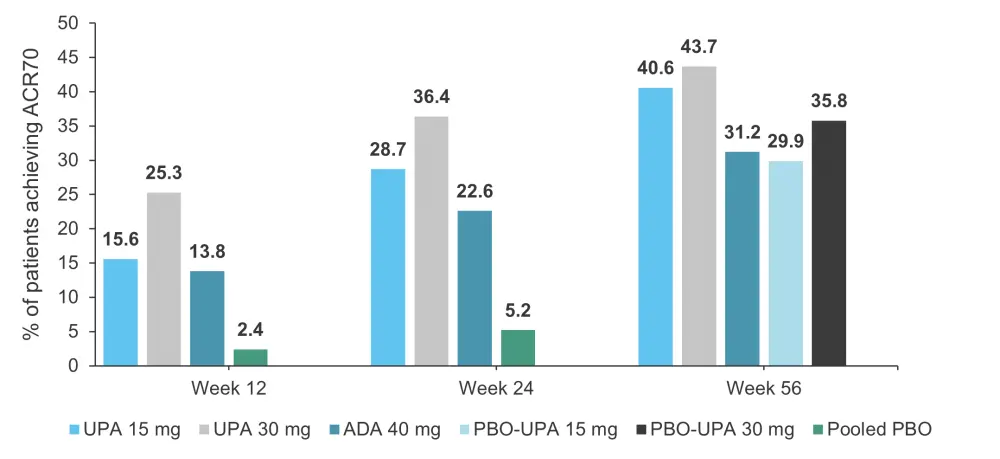
ACR70, 70% improvement in American College of Rheumatology response criteria; ADA, adalimumab; UPA, upadacitinib; PBO, placebo.
*Adapted from McInnes, et al.1
All ACR components were improved from baseline across all cohorts. Patients who switched from placebo to upadacitinib had comparable results to those originally randomized to upadacitinib (Table 2).
Table 2. ACR components and patient-reported outcomes at 56 weeks*
|
ACR, American College of Rheumatology; ADA, adalimumab; DAPSA, Disease Activity in Psoriatic Arthritis; EOW, every other week; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability Index; MCS, Mental Component Summary; MMRM, mixed-effects model repeated measures; NRS, numeric rating scale; PCS, Physical Component Summary; PhGA, Physicians’ Global Assessment of Disease Activity; PtGA, Patients' Global Assessment of Disease Activity; QD, once daily; SF-36, Short Form Health Survey questionnaire; SJC66 swollen joint count of 66 joints; TJC68, tender joint count of 68 joints; UPA, upadacitinib; WPAI, Work Productivity and Activity Impairment. |
|||||
|
Parameter |
Placebo-UPA 15 mg QD |
Placebo-UPA 30 mg QD |
UPA 15 mg QD |
UPA 30 mg QD |
ADA 40 mg EOW |
|---|---|---|---|---|---|
|
PhGA |
−4.7 (−4.9 to −4.5) |
−4.8 (−5.1 to −4.6) |
−4.9 (−5.0 to −4.7) |
−4.9 (−5.0 to −4.7) |
−4.7 (−4.8 to −4.5) |
|
SJC66 |
−9.2 (−9.7 to −8.7) |
−9.6 (−10.1 to −9.2) |
−9.7 (−10.1 to −9.4) |
−9.8 (−10.1 to −9.4) |
−9.6 (−9.9 to −9.3) |
|
TJC68 |
−15.4 (−16.4 to −14.3) |
−15.6 (−16.7 to −14.6) |
−15.7 (−16.4 to −14.9) |
−15.8 (−16.5 to −15.0) |
−15.3 (−16.1 to −14.6) |
|
C-reactive protein |
−7.5 (−8.6 to −6.5) |
−8.7 (−9.8 to −7.7) |
−7.8 (−8.6 to −7.1) |
−8.6 (−9.3 to −7.9)‡ |
−7.5 (−8.2 to −6.7) |
|
HAQ-DI |
−0.40 (−0.48 to −0.32) |
−0.45 (−0.53 to −0.38) |
−0.54 (−0.59 to −0.48)§ |
−0.56 (−0.61 to −0.50)‡ |
−0.43 (−0.49 to −0.38) |
|
FACIT-F |
7.2 (5.9 to 8.6) |
8.8 (7.4 to 10.1) |
8.9 (8.0 to 9.9) |
8.4 (7.4 to 9.4) |
7.6 (6.7 to 8.6) |
|
SF-36 PCS |
9.3 (8.0 to 10.5) |
9.8 (8.6 to 11.1) |
10.8 (10.0 to 11.7)§ |
10.5 (9.6 to 11.4)‡ |
8.9 (8.0 to 9.8) |
|
SF-36 MCS |
3.6 (2.3 to 4.9) |
4.0 (2.7 to 5.3) |
5.2 (4.2 to 6.1) |
4.4 (3.4 to 5.3) |
4.3 (3.4 to 5.2) |
|
Self-assessment of psoriasis symptoms |
−28.1 (−30.5 to −25.6) |
−30.3 (−32.8 to −27.9) |
−29.6 (−31.4 to −27.9)§ |
−30.4 (−32.2 to −28.7)‡ |
−25.8 (−27.6 to −24.1) |
|
WPAI overall work impairment‖
|
−21.9 (−27.1 to −16.7) |
−24.7 (−30.0 to −19.5) |
−24.5 (−28.1 to −20.9) |
−22.9 (−26.5 to −19.3) |
−20.8 (−24.5 to −17.1) |
|
PtGA (NRS) |
−3.4 (−3.7 to −3.0) |
−3.7 (−4.0 to −3.3) |
−3.7 (−3.9 to −3.4)§ |
−3.6 (−3.9 to −3.4)‡ |
−3.2 (−3.4 to −2.9) |
|
Patients’ assessment of pain (NRS) |
−3.1 (−3.5 to −2.8) |
−3.3 (−3.7 to −3.0) |
−3.3 (−3.6 to −3.1)§
|
−3.3 (−3.6 to −3.1)‡ |
−2.9 (−3.1 to −2.7) |
|
DAPSA |
−38.9 (−41.1 to −36.7) |
−41.6 (−43.8 to −39.3) |
−40.0 (−41.5 to −38.4) |
−41.0 (−42.6 to −39.4)‡ |
−38.5 (−40.1 to −36.9) |
- In all treatment groups, the proportion of patients achieving Psoriatic Arthritis Response Criteria (PsARC) was also maintained from Week 24 to Week 56.
-
- At Week 56, more patients achieved PsARC response with upadacitinib 30 mg compared with adalimumab (nominal p ≤ 0.05).
- Based on linear extrapolation at Week 56, mean changes from baseline in radiographic endpoints were comparable between both upadacitinib cohorts and the adalimumab cohort.
- MDA responses can be seen in Figure 3.
- For those originally randomized to upadacitinib or adalimumab, the proportion of patients achieving MDA were maintained from Week 24 to Week 56.
- Overall, more patients in the upadacitinib 30 mg cohort achieved MDA compared with the adalimumab cohort (nominal p ≤ 0.05).
- For the placebo cohorts, the percentage of patients achieving MDA increased when they were switched to upadacitinib.
- Improvements in the skin outcomes Psoriasis Area and Severity Index (PASI75/90/100) and Static Investigator Global Assessment of Psoriasis of 0 or 1 (sIGA 0/1) response rates were maintained over time.
- After patients switched to upadacitinib from placebo, the proportion of patients achieving PASI75/90/100 and sIGA 0/1 increased and were similar to the upadacitinib cohorts by Week 36.
Figure 3. Percentage of patients achieving MDA (NRI)*†
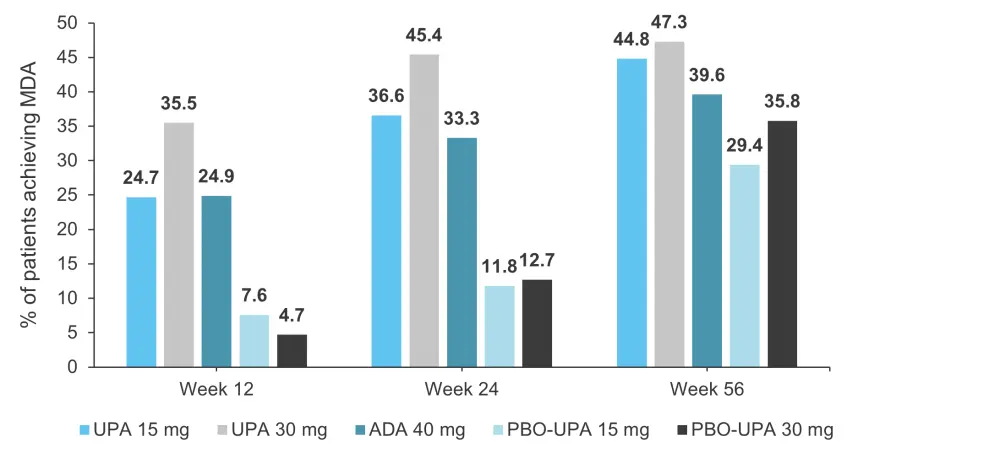
ADA, adalimumab; MDA, minimal disease activity; NRI, non-responder imputation; PBO, placebo; UPA, upadacitinib.
*Adapted from McInnes, et al.1
†NRI with additional rescue handling was used, where patients rescued at Week 16 are imputed as non-responders.
Patient-reported outcomes
Improvements in Health Assessment Questionnaire-Disability Index (HAQ-DI), Functional Assessment of Chronic Illness Therapy-Fatigue, Short Form Health Survey questionnaire (SF-36), Physical Component Summary (PCS) and Mental Component Summary (MCS), patients’ assessment of pain, Patients’ Global Assessment of Disease Activity (PtGA), and Work Productivity and Activity Impairment (WPA) were maintained from Week 24 to Week 56.
Patient reported outcomes at Week 56 are detailed in Table 2.
Safety
- At Week 56, treatment-emergent adverse event (TEAE) rates were highest in the 30 mg upadacitinib cohort (333.9 events/100 patient years vs 281.1 [UPA 15 mg] and 265.9 [ADA]).
- The most frequent AEs were upper respiratory tract infection and blood creatine phosphokinase elevations.
- Exposure-adjusted event rates of TEAEs at Week 56 can be seen in Table 3.
- Of note, patients in the UPA 30 mg cohort had higher rates of infection.
- Event rates of malignancy were similar across the upadacitinib and adalimumab treatment groups; no notable malignancy patterns or types were observed.
Table 3. Exposure-adjusted event rates of treatment-emergent AEs at Week 56.
|
ADA, adalimumab; AE, adverse event; CPK, creatine phosphokinase; GI, gastrointestinal; PY, patient years, UPA, upadacitinib. |
|||
|
AE, events/100 PY |
UPA 15 mg |
UPA 30 mg |
ADA 40 mg |
|---|---|---|---|
|
Infection |
94.9 |
114.3 |
71.1 |
|
Serious infection |
2.9 |
4.7 |
1.3 |
|
Opportunistic infection |
0.4 |
0.8 |
0.0 |
|
Herpes zoster |
3.9 |
6.4 |
0.5 |
|
GI perforation |
0.2 |
0.0 |
0.0 |
|
Hepatic disorder |
19.1 |
22.2 |
24.9 |
|
Anemia |
3.0 |
6.2 |
1.6 |
|
Neutropenia |
2.4 |
6.4 |
4.3 |
|
Lymphopenia |
3.2 |
4.0 |
0.2 |
|
CPK elevation |
11.9 |
17.3 |
7.3 |
|
Renal dysfunction |
0.2 |
0.2 |
0.0 |
- Deaths were reported in
- two patients in the upadacitinib 15 mg cohort (due to metastatic lung cancer and lower respiratory tract infection);
- two patients in the upadacitinib 30 mg cohort (due to coronavirus infection and interstitial lung disease);
- one patient in the adalimumab cohort (due to a traffic accident); and
- one patient in the placebo group (due to an unspecified emergency while driving).
Conclusion
The 56-week efficacy data across various domains of PsA support the benefit of continued upadacitinib therapy in patients with active PsA and inadequate response or intolerance to ≥1 non-biological DMARD. Moreover, the 56-week safety data were comparable to findings from Week 24. However, a major limitation of this trial includes the lack of axial imaging to assess for psoriatic spondylitis. As the diagnosis was made on presumptive criteria, patients without true spondylitis may have been included. Furthermore, the study was not powered or designed to include a prespecified statistical comparison for efficacy between the upadacitinib arms and adalimumab arm through to Week 56.
Despite its limitations, the results of this trial along with those of the SELECT-PSA 2 trial lead the National Institute for Health and Care Excellence (NICE) to recommend upadacitinib alone or in combination with methotrexate as a treatment option for adult patients with active PsA whose disease has not responded well enough to DMARDs or those that cannot tolerate them. This recommendation was published earlier this year and is only for patients who have peripheral arthritis with ≥3 tender joints and ≥3 swollen joints and
- have had two conventional DMARDs and ≥1 biological DMARD; or
- where TNF-alpha inhibitors are contraindicated.2
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

