All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Patient-reported outcomes from the SELECT-PSA-2 phase III randomized control trial investigating oral upadacitinib for the treatment of psoriatic arthritis
Psoriatic arthritis (PsA) is an inflammatory disease that can present with multiple clinical manifestations, such as plaque psoriasis, arthritis, dactylitis, enthesitis, and axial skeleton involvement.1 PsA can also significantly impact a patient’s quality of life, including physical and emotional functioning, the ability to perform daily activities, and work productivity. Treatment options for patients with PsA currently include disease-modifying antirheumatic drugs (DMARDs), such as methotrexate; biological DMARDs (bDMARDs), including inhibitors of tumor necrosis factor and interleukins; and targeted non-biological DMARDs (non-bDMARDs), such as Janus kinase (JAK) inhibitors. However, adequate disease control is still hard to reach in some patients; therefore, new and effective treatment options are much needed.1
Upadacitinib is an oral, reversible, JAK inhibitor that is approved in Europe for patients with PsA and ankylosing spondylitis with an inadequate response or an intolerance to DMARDs and conventional therapy.1 In February 2022, the National Institute for Health and Care Excellence (NICE) approved upadacitinib, as monotherapy or with methotrexate, for the treatment of PsA in patients who have not responded to treatment, are intolerant to ≥2 conventional DMARDS or ≥1 bDMARD, or are contraindicated to receive tumor necrosis factor-α inhibitors.2 NICE also state that it is recommended only if patients have peripheral arthritis with ≥3 tender joints or ≥3 swollen joints.2
The SELECT-PsA 2 trial (NCT03104374) investigated the effect of upadacitinib compared with placebo in adult patients with active PsA and an inadequate response or intolerance to ≥1 bDMARD.1 Patient-reported outcomes (PROs) from this trial were published by Strand et al.1 in Rheumatology and Therapy, and a summary of these results is provided below.
Study design and patient characteristics
This was a phase III, multicenter, randomized, placebo-controlled trial (Figure 1). Patients were excluded from this study if they had prior exposure to JAK inhibitors or were receiving current treatment with ≥2 non-bDMARDs.
Figure 1. Study design*
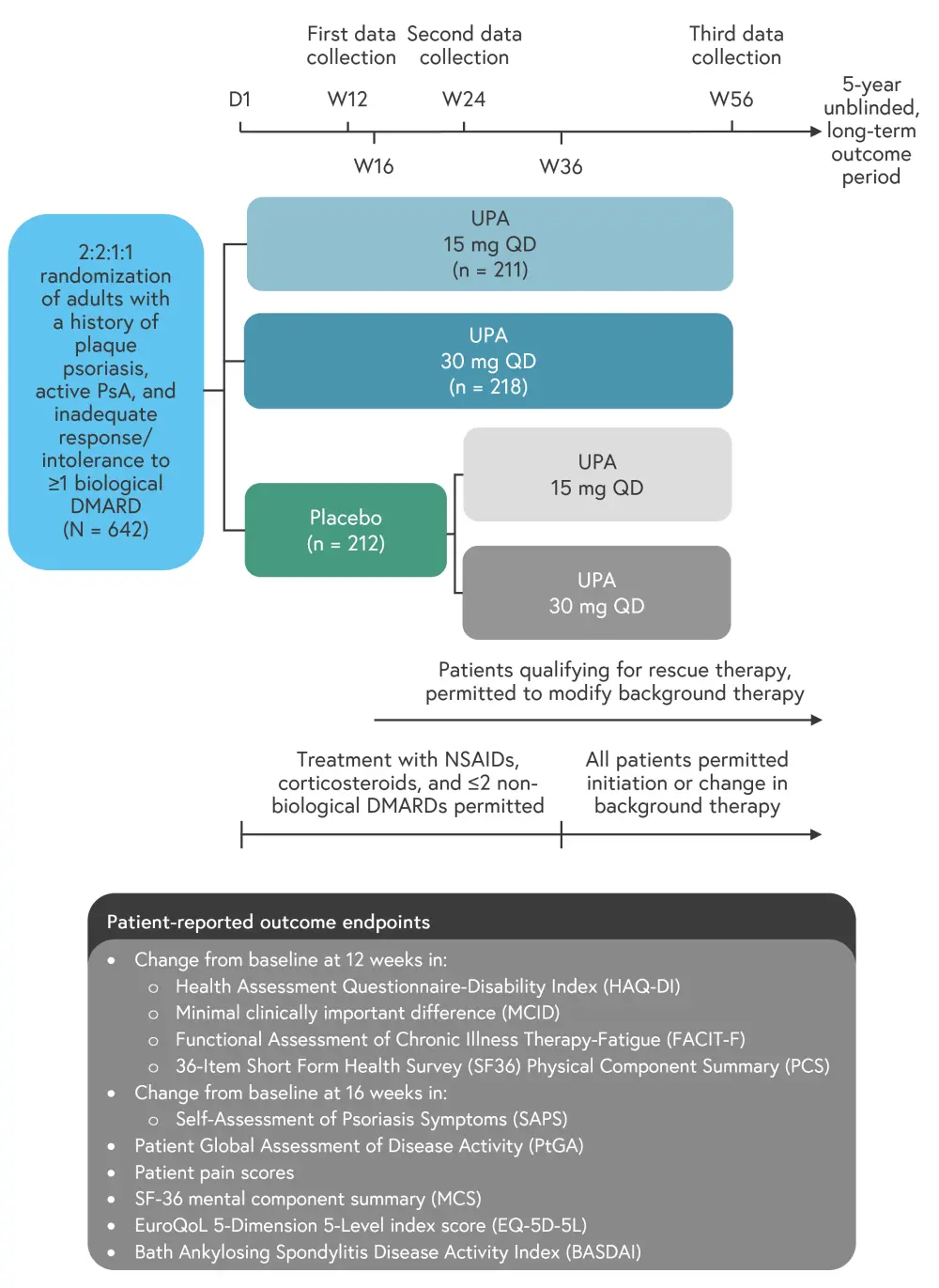
D, day; DMARD, disease-modifying antirheumatic drug; NSAID, non-steroidal anti-inflammatory drug; PsA, psoriatic arthritis; QD, once daily; UPA, upadacitinib; W, week.
*Data from Strand, et al.1
Baseline characteristics were well balanced, as can be seen in Table 1.
Table 1. Baseline patient characteristics*
|
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; bDMARD, biological disease modifying anti-rheumatic drug; DMARD, disease modifying anti-rheumatic drug; EQ-5D-5L, EuroQoL 5-Dimension 5-Level index score; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability Index; PsA, psoriatic arthritis; PtGA, Patient Global Assessment Of Disease Activity; SF36 PCS, 36-Item Short Form Health Survey Physical Component Summary score; UPA, upadacitinib. |
|||
|
Characteristic, % (unless otherwise stated) |
Placebo |
UPA 15 mg |
UPA 30 mg |
|---|---|---|---|
|
Female |
56.6 |
53.6 |
52.8 |
|
Mean age, years |
54.1 |
53.0 |
53.0 |
|
White race |
87.7 |
86.7 |
89.9 |
|
Mean duration since PsA diagnosis, years |
11.0 |
9.6 |
9.7 |
|
No of prior failed DMARDS |
|
|
|
|
0 |
8.5 |
7.6 |
7.8 |
|
1 |
63.7 |
59.7 |
59.6 |
|
2 |
16.5 |
16.6 |
21.1 |
|
≥3 |
11.3 |
16.1 |
11.5 |
|
Use of ≥1 non-bDMARD at baseline |
47.2 |
46.4 |
45.0 |
|
Presence of dactylitis† |
30.2 |
26.1 |
22.9 |
|
Presence of enthesitis‡ |
67.9 |
63.0 |
69.7 |
|
Mean PtGA 0-10, numerical rating scale |
6.8 |
6.8 |
6.7 |
|
Mean pain 0-10, numerical rating scale |
6.6 |
6.4 |
6.2 |
|
Mean HAQ-DI |
1.23 |
1.10 |
1.19 |
|
Mean FACIT-F |
26.6 |
27.6 |
28.8 |
|
Mean SF-36 PCS |
34.5 |
35.0 |
34.8 |
|
Mean EQ-5D-5L |
0.59 |
0.61 |
0.61 |
|
Mean BASDAI |
6.1 |
5.9 |
0.61 |
Results
For PtGA, pain, and HAQ-DI, improvements were reported as early as Week 2 of treatment in both upadacitinib treatment groups (Figure 2). When compared with placebo, significant improvements from baseline were observed for all key PROs at Weeks 12 and 24, and these were maintained or improved further through to Week 56 (Figure 3).
Figure 2. Percentage of patients reporting a minimum clinically important difference for key PROs at 2 weeks of treatment*†
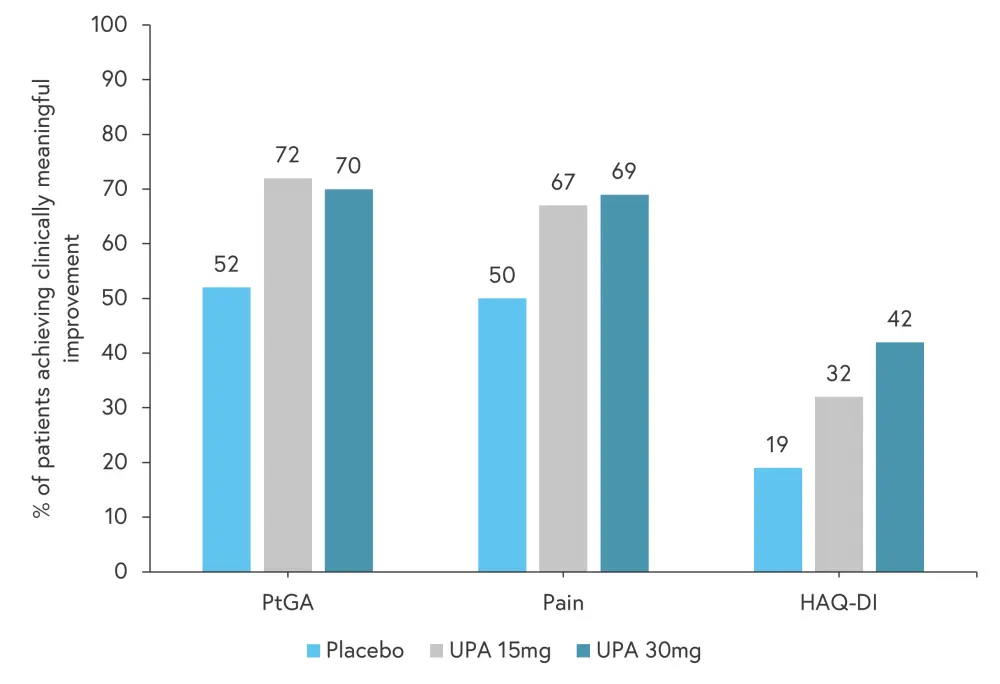
HAQ-DI, Health Assessment Questionnaire-Disability Index; MCID, minimum clinically important difference; PtGA, Patient Global Assessment Of Disease Activity; UPA, upadacitinib.
*Data from Strand, et al.1
†p ≤ 0.01 versus placebo for all PROs at UPA 15 mg and UPA 30 mg.
Figure 3. Improvements in patient-reported outcome scores at Weeks 12, 24, and 56*†
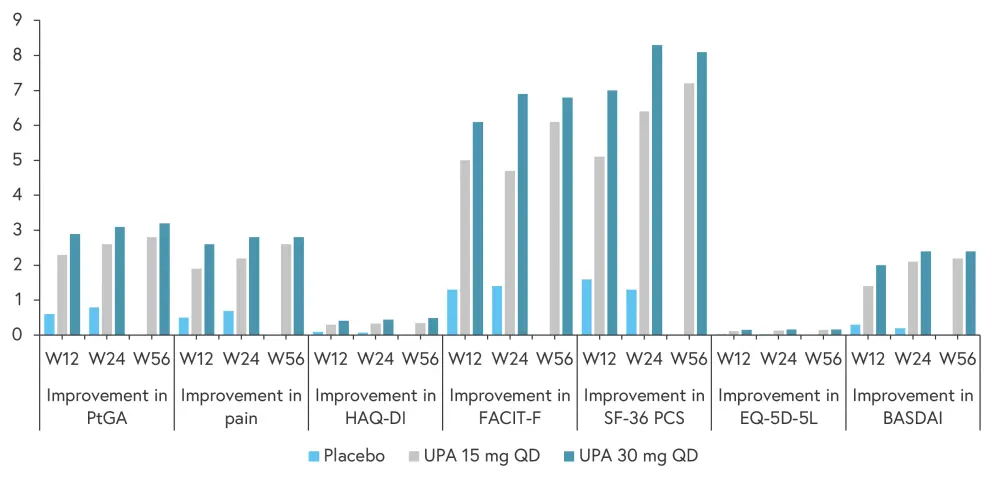
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; EQ-5D-5L, EuroQoL 5-Dimension 5-Level index score; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability Index; PtGA, Patient Global Assessment Of Disease Activity; SF36 PCS, 36-Item Short Form Health Survey Physical Component Summary score; UPA, upadacitinib; W, week.
*Adapted from Strand, et al.1
†p ≤ 0.001 versus placebo for all patient reported outcomes at Weeks 12 and 24.
Patients treated with placebo who switched to upadacitinib at Week 24 experienced rapid improvements in PtGA, pain HAQ-DI, and FACIT-F scores, with similar scores to those seen in the upadacitinib treatment groups at Week 56.
A 50% improvement in initial BASDAI score (BASDAI50) was observed in significantly more patients treated with upadacitinib than those patients treated with placebo (p < 0.05). The frequency of BASDAI50 was
- 20% at 12 weeks and 32% at 24 weeks in the 15 mg upadacitinib cohort;
- 29% at 12 weeks and 35% at 24 weeks in the 30 mg upadacitinib cohort; and
- 7% at 12 weeks and 4% at 24 weeks in the placebo cohort.
In terms of clinically meaningful improvements in PROs, a significantly greater proportion of patients in the upadacitinib treatment arms experienced at least a minimal clinically important difference across most PROs, with greatest improvements seen in PtGA and pain (Figure 4).
Figure 4. Proportion of patients reporting improvement in ≥MCID in patient-reported outcomes at Week 12*†
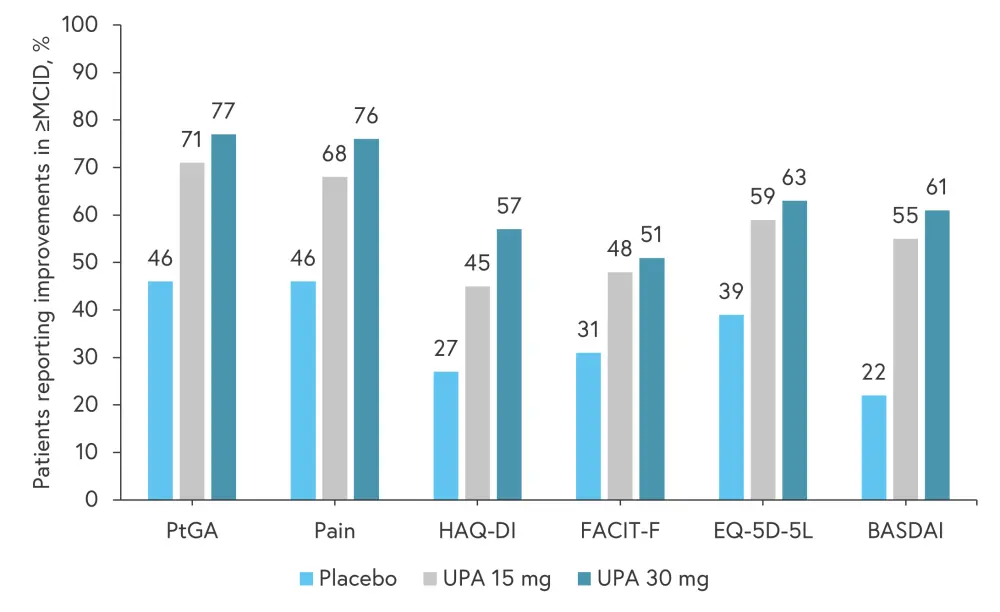
≥MCID, at least a minimal clinically important difference; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; EQ-5D-5L, EuroQoL 5-Dimension 5-Level index score; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability Index; PtGA, Patient Global Assessment Of Disease Activity; UPA, upadacitinib.
*Adapted from Strand, et al.1
†Nominal p ≤ 0.001 versus placebo for all PROs at UPA 15 mg and UPA 30 mg.
Conclusion
The results from this SELECT-PSA 2 post hoc analysis demonstrate that treatment with upadacitinib can offer significant improvements in the quality of life of patients with PsA. These treatment benefits are realized as early as 2 weeks into treatment initiation and are maintained at >12 months. Limitations of this study include the lack of placebo or comparator after 24 weeks, and that it was not powered to detect differences between the two upadacitinib treatment arms.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

