All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
The safety and efficacy of tildrakizumab in patients with psoriasis and concurrent metabolic syndrome
The metabolic syndrome (MetS) is characterized by the co-occurrence of several known cardiovascular risk factors such as obesity, dyslipidemia, and hypertension; patients with MetS are at an increased risk of stroke, diabetes, and cardiovascular disease.
Patients with psoriasis are at increased risk of metabolic disorders, including MetS, and associated mortalities compared with the general population.1 Additionally, some treatments for psoriasis may be less effective in patients with MetS, although there is a lack of long-term safety and efficacy data to support this.1
The phase III randomized reSURFACE 1 (NCT01722331) and reSURFACE 2 (NCT01729754) trials investigated the efficacy and safety of tildrakizumab (TIL) in patients with psoriasis, both with and without concurrent MetS.1 Here, we discuss a post-hoc analysis of the 5-year data from reSURFACE 1 and reSURFACE 2 published in Journal of the European Academy of Dermatology and Venereology: JEADV by Fernandez, et al.1
Study design1
This was a post-hoc analysis of the phase III reSURFACE 1 and reSURFACE 2 clinical trials. Patients in both trials who achieved at least a 50% improvement from baseline in Psoriasis Area and Severity Index (PASI) score, by Week 62 for reSURFACE 1 or Week 52 for reSURFACE 2, were eligible to enter an optional long-term extension period of up to 5 years.
Classification of MetS was based on patients having ≥3 of the following characteristics: BMI >30 kg/m2, triglycerides ≥150 mg/dL, high-density lipoprotein cholesterol (<40 mg/dL in men or >50 mg/dL in women), systolic blood pressure of ≥130 mm Hg or diastolic blood pressure of ≥85 mm Hg, or fasting glucose ≥110 mg/dL.
Efficacy outcomes were measured by changes in PASI scores, with the proportion of patients achieving a ≥75%, ≥90%, and ≥100% improvement from baseline PASI score evaluated every 12 weeks until Week 148, then every 24 weeks after this. Safety outcomes was measured as exposure-adjusted incidence rates (EAIRs) of treatment-emergent adverse events (TEAEs), Tier 1 TEAEs, and serious AEs (SAEs).
Results
Baseline patient characteristics are shown in Table 1.
Table 1. Baseline patient characteristics in reSURFACE 1 and reSURFACE 2*
|
BMI, body mass index; BSA, body surface area; CV, cardiovascular; MetS, metabolic syndrome; PASI, Psoriasis Area and Severity Index; PGA, physician global assessment; PsA, psoriatic arthritis; TIL, tildrakizumab. |
||||||||
|
Characteristic |
reSURFACE 1 |
reSURFACE 2 |
||||||
|---|---|---|---|---|---|---|---|---|
|
TIL 100 mg |
TIL 200 mg |
TIL 100 mg |
TIL 200 mg |
|||||
|
Without |
With |
Without |
With |
Without |
With |
Without |
With |
|
|
n |
98 |
26 |
111 |
34 |
167 |
44 |
130 |
30 |
|
Age, years |
46.1 |
49.1 |
46.1 |
50.7 |
43.1 |
45.9 |
44.5 |
48.7 |
|
Male |
66.3 |
69.2 |
71.2 |
55.9 |
71.3 |
77.3 |
63.1 |
76.7 |
|
White |
65.3 |
80.8 |
60.4 |
79.4 |
91.6 |
93.2 |
90.8 |
96.7 |
|
BMI, kg/m2 |
27.9 |
35.6 |
28.4 |
38.5 |
27.4 |
35.6 |
27.3 |
37.6 |
|
Affected BSA |
29.4 |
32.2 |
32.1 |
30.1 |
33.7 |
30.3 |
31.3 |
27.6 |
|
Disease |
17.2 |
16.2 |
16.7 |
16.7 |
15.9 |
15.2 |
18.0 |
20.1 |
|
Baseline PASI |
19.9 |
20.5 |
21.3 |
20.6 |
19.5 |
20.8 |
19.5 |
19.2 |
|
Baseline PGA |
3.3 |
3.3 |
3.4 |
3.5 |
3.3 |
3.4 |
3.3 |
3.4 |
|
CV disorders |
14.3 |
65.4 |
27.0 |
47.1 |
17.4 |
38.6 |
20.8 |
56.7 |
|
Diabetes |
8.2 |
30.8 |
9.9 |
23.5 |
3.0 |
15.9 |
8.5 |
23.3 |
|
PsA |
16.3 |
19.2 |
16.2 |
26.5 |
14.4 |
25.0 |
13.1 |
13.3 |
|
Response to |
44.9 |
71.4 |
66.1 |
57.1 |
64.7 |
54.5 |
61.5 |
56.7 |
|
Prior biologic |
16.3 |
30.8 |
18.0 |
20.6 |
13.2 |
11.4 |
13.1 |
23.3 |
Overall, 269 patients entered the extension phase of the reSURFACE 1 trial. This included 124 patients treated continuously with TIL 100 mg (26 with MetS and 98 without MetS), of which 20 patients with MeTS and 72 patients without MeTS reached Week 256 of treatment, and 145 patients treated with TIL 200 mg (34 with MetS and 111 without MetS), of which 31 patients with MetS and 83 patients without MetS reached Week 256 of treatment.
In the reSURFACE 2 trial, 371 patients entered the extension phase. This included 211 patients treated with TIL 100 mg (44 with MetS and 167 without MetS), of which 22 patients with MetS and 130 patients without MetS completed treatment to Week 244, and 160 patients treated with TIL 200 mg (30 with MetS and 130 without MetS), of which 36 patients with MetS and 130 patients without MetS completed treatment to Week 244.
Efficacy
The median change in PASI score from Week 28 to Week 244 in reSURFACE 1 and reSURFACE 2 was similar, both in patients with and without MetS and for both doses of TIL. In both studies, median reduction from baseline PASI score at all time points in patients with vs without MetS was >83% vs >89% for TIL 100 mg and >85% vs >90% for TIL 200 mg. For patients receiving TIL 100 mg, the median reduction in PASI score ranged from 83–92% between Week 28 and Week 244, resulting in the majority of patients achieving an absolute PASI score <3. Among patients receiving TIL 200 mg, the median reduction from baseline PASI score was >85% for both trials. The percentage of patients achieving PASI75 is shown in Figure 1.
Figure 1. PASI75 at A Week 28 and B Week 244*
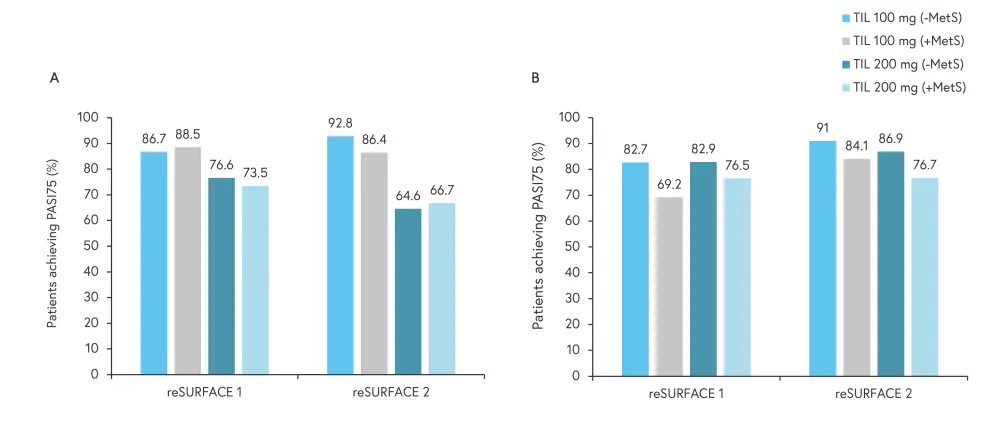
MetS, metabolic syndrome; PASI75, 75% reduction in Psoriasis Area and Severity Index score; TIL, tildrakizumab.
*Adapted from Fernandez, et al.1
At Week 244, numerically fewer patients with MetS in both trials achieved PASI75, compared with patients without MetS, regardless of TIL dose.
Safety
The pooled data from reSURFACE 1 and reSURFACE 2 showed both doses of TIL had a safety profile similar to what has been previously reported. The rate of Tier 1 AEs was highest in patients with MetS treated with TIL 200 mg, with the most common being infections and infestations, malignancies excluding melanoma, and nonmelanoma skin cancer; the number of SAEs were slightly higher in patients with MetS. The rate of SAEs, defined using the Medical Dictionary for Regulatory Activities System Organ Class codes, is shown in Figure 2.
Figure 2. Rate of SAEs and most common SAEs*
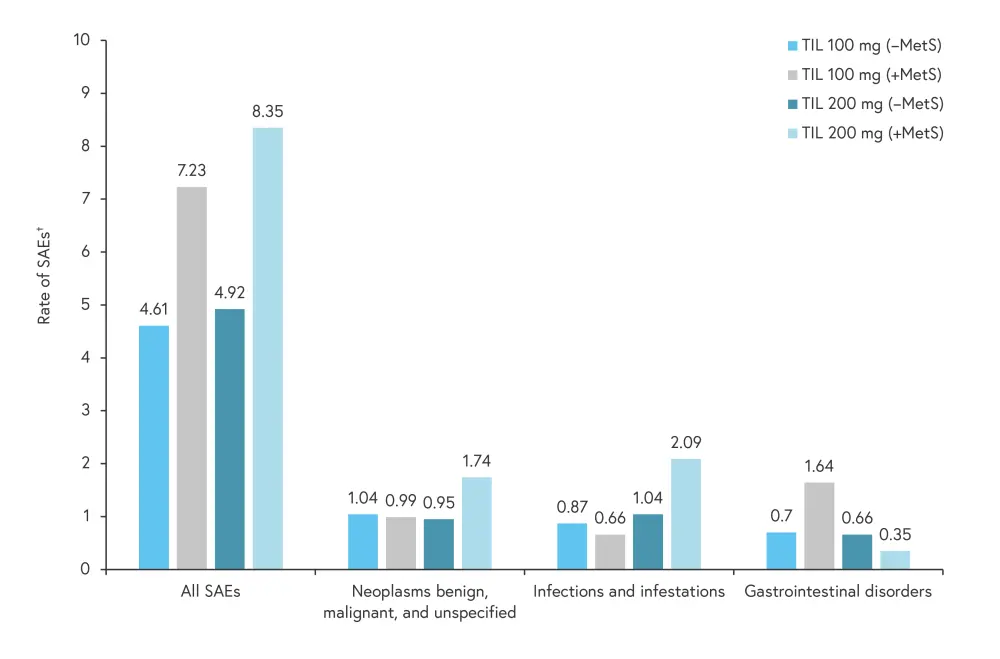
MetS, metabolic syndrome; SAE, serious adverse event; TIL, tildrakizumab.
*Adapted from Fernandez, et al.1
†SAE rate is an exposure-adjusted rate as number of patients with the event per 100 patient years of exposure.
Overall, 10 patients discontinued treatment due to AEs in reSURFACE 1, including one on TIL 100 mg and three on TIL 200 mg; none had MetS. In reSURFACE 2, 12 patients discontinued treatment due to AEs (one patient with MetS and four patients without MetS on TIL 100 mg; four patients with MetS and three patients without MetS on TIL 200 mg).
Conclusion
These findings demonstrate the similar short- and long-term efficacy of TIL in patients with psoriasis, with or without MetS, for up to 5 years. The safety of TIL was also similar in patients with and without MetS; however, the rate of SAEs was higher in patients with MetS. Although it has been suggested that use of biologic agents increases cancer risk, this analysis reported no evidence for a higher risk of cancer or serious infection with TIL across patients with or without MetS. Further studies utilizing other biologic agents, with equal proportions of patients with and without MetS, will further elucidate the impact of MetS in patients with psoriasis.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with psoriatic arthritis do you see per month?

