All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
The effect of race/ethnicity on treatment safety and efficacy in plaque psoriasis
Psoriasis affects an estimated 7.4 million people in the United States alone, with the majority of patients being White; however, it is also one of the most common skin conditions diagnosed in patients of color. Historically, most patients enrolled on psoriasis clinical trials have been White, this has led to a lack of safety and efficacy data available for patients of color on many psoriasis treatments.
A systemic review by Ferguson et al.1 evaluated the differences in efficacy and safety of 12 psoriasis treatments based on patient race/ethnicity; although data for each race/ethnicity was not available in some studies.
Study design
Studies were identified from PubMed, Embase, Web of Science, and EBSCO databases. All patients in the included 24 studies had plaque psoriasis; the search design in shown in Figure 1. Studies had similar inclusion criteria including ≥10% body surface area affected, a Psoriasis Area Severity Index (PASI) ≥12, and static Physician’s Global Assessment, Physician’s Global Assessment, or Investigator’s Global Assessment ≥3. All patients in the studies self-categorized their race/ethnicity.
Figure 1. Search design and inclusion criteria*
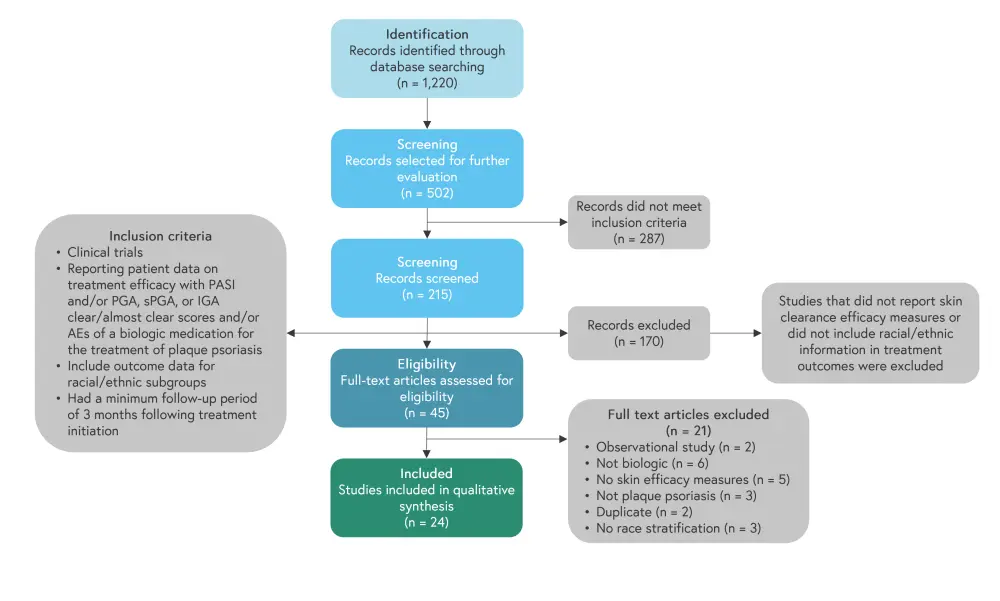
*Adapted from Ferguson, et al.1
Results
Baseline patient characteristics are shown in Table 1. The majority of patients were White, with Asian patients having the highest mean PASI score and lowest mean Dermatology Life Quality Index of all the race/ethnicities included.
Table 1. Baseline patient characteristics*
|
BMI, body mass index; BSA, body surface area affected; DLQI, Dermatology Life Quality Index; NR, not reported; PASI, Psoriasis Area and Severity Index. |
|||||
|
Characteristic, % |
Race/ethnicity |
||||
|---|---|---|---|---|---|
|
White |
Asian |
Latino |
Black |
Other |
|
|
Total patients, n |
9,745 |
2,740 |
728 |
138 |
140 |
|
Mean age, years |
45 |
43.7 |
44.7 |
44.8 |
44.2 |
|
Male |
68 |
76.5 |
46.4 |
63.9 |
70.0 |
|
Mean BMI |
30.1 |
20.7 |
29.9 |
31.0 |
29.3 |
|
Medication |
|
|
|
|
|
|
Ixekizumab |
23.2 |
4.1 |
9.9 |
0 |
0 |
|
Secukinumab |
1.9 |
2.7 |
25.6 |
0 |
0 |
|
Brodalumab |
11.4 |
6.9 |
18.1 |
26.1 |
0 |
|
Ustekinumab |
5.7 |
29.9 |
9.3 |
14.5 |
0 |
|
Guselkumab |
6.9 |
4.5 |
0 |
8.7 |
14.2 |
|
Adalimumab |
22.6 |
26.6 |
0 |
17.4 |
12.9 |
|
Infliximab |
0 |
4.7 |
0 |
0 |
0 |
|
Etanercept |
3.9 |
0 |
11.1 |
0 |
0 |
|
Certolizumab |
8.7 |
0 |
0 |
0 |
0 |
|
Tofacitinib |
15.7 |
16.2 |
0 |
33.3 |
72.9 |
|
Tildrakizumab |
0 |
4.4 |
0 |
0 |
0 |
|
Efalizumab |
0 |
0 |
26.0 |
0 |
0 |
|
BSA |
27.0 |
37.1 |
32.7 |
28.8 |
27.0 |
|
Mean PASI score |
20.4 |
24.9 |
23.3 |
21.8 |
21.2 |
|
Mean DLQI score |
13.3 |
12.4 |
14.5 |
16.5 |
NR |
Efficacy of treatments
Efficacy was reported at Week 12 except for one adalimumab study, one study of guselkumab, and two studies of tofacitinib which evaluated efficacy at Week 16. The percentage of clear or almost clear responses to treatment are shown in Figure 2.
Figure 2. Drug efficacy at Week 12 in percentage responders for 2A ustekinumab, 2B ixekizumab, 2C guselkumab, 2D brodalumab, 2E secukinumab, 2F adalimumab, 2G etanercept, 2H efalizumab, 2I certolizumab, and 2J infliximab*
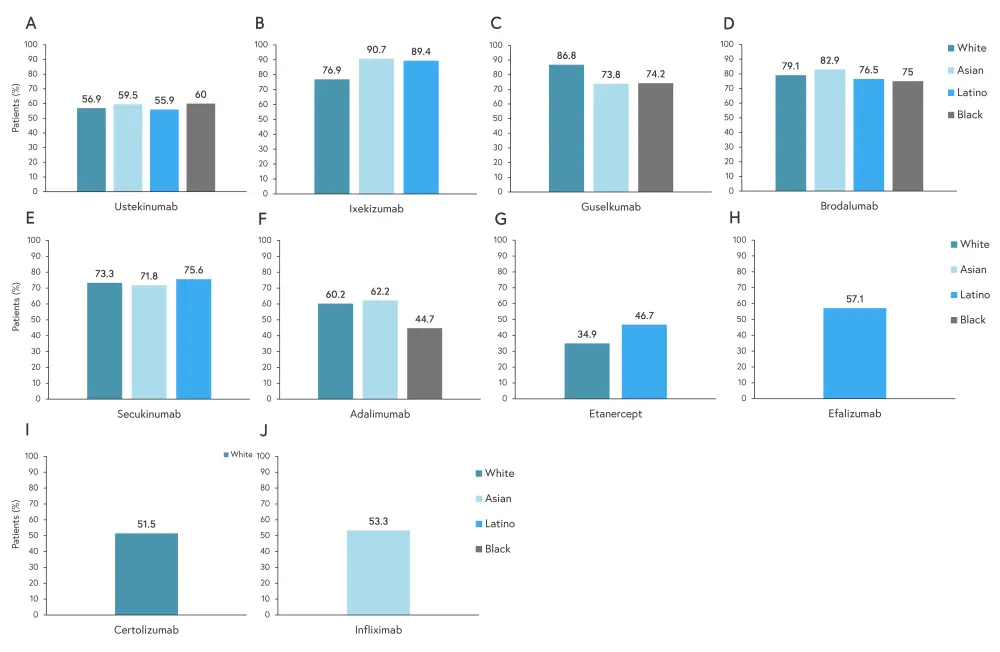
*Adapted from Ferguson, et al.1
The percentage of patients who achieved a 75% improvement in PASI (PASI75) are shown in Figure 3. Ixekizumab showed the greatest percentage of patients achieving PASI75 in Asian and Latino patients. Brodalumab showed the greatest percentage of patients achieving PASI75 in White and Black patients.
Figure 3. Patients achieving PASI75 with 2A ustekinumab, 2B brodalumab, 2C ixekizumab, 2D tofacitinib, 2E secukinumab, 2F adalimumab, 2G etanercept, 2H efalizumab, 2I certolizumab, and 2J infliximab treatment*
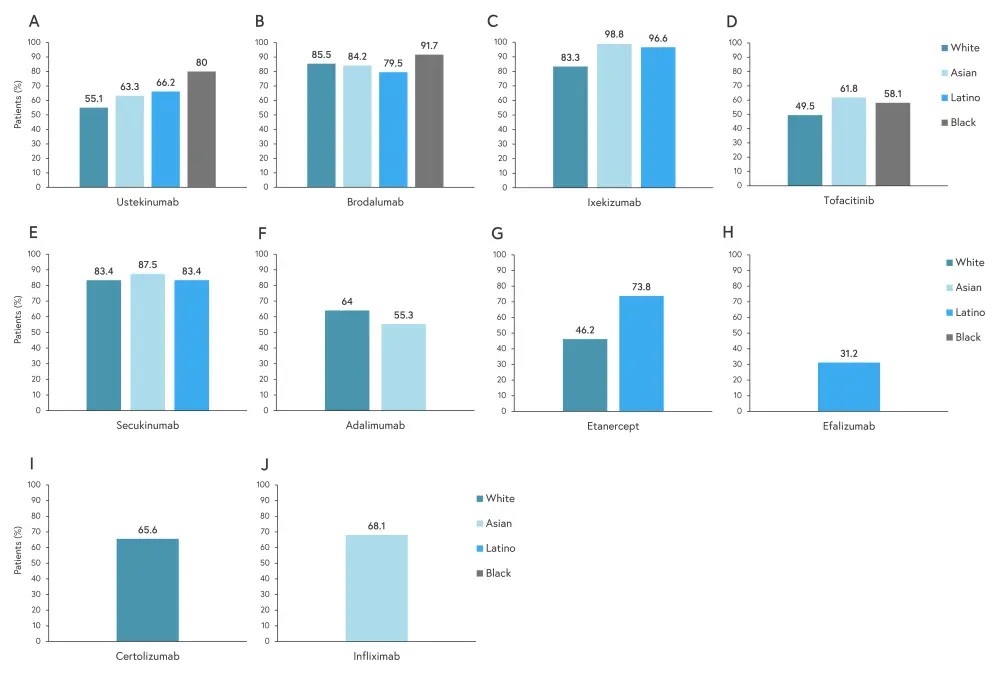
PASI75, 75% improvement in Psoriasis Area and Severity Index.
*Adapted from Ferguson, et al.1
Safety of treatments
The incidence of adverse events (AEs) was reported upon study completion, with study periods ranging from 12 to 192 weeks. Rates of AEs, severe AEs, and drug discontinuation are shown in Figure 4.
Figure 4. Rate of AEs, severe AEs, and drug discontinuations for 3A ixekizumab, 3B secukinumab, 3C etanercept, 3D adalimumab, 3E tildrakizumab, 3F certolizumab, 3G tofacitinib, 3H efalizumab, and 2I infliximab*
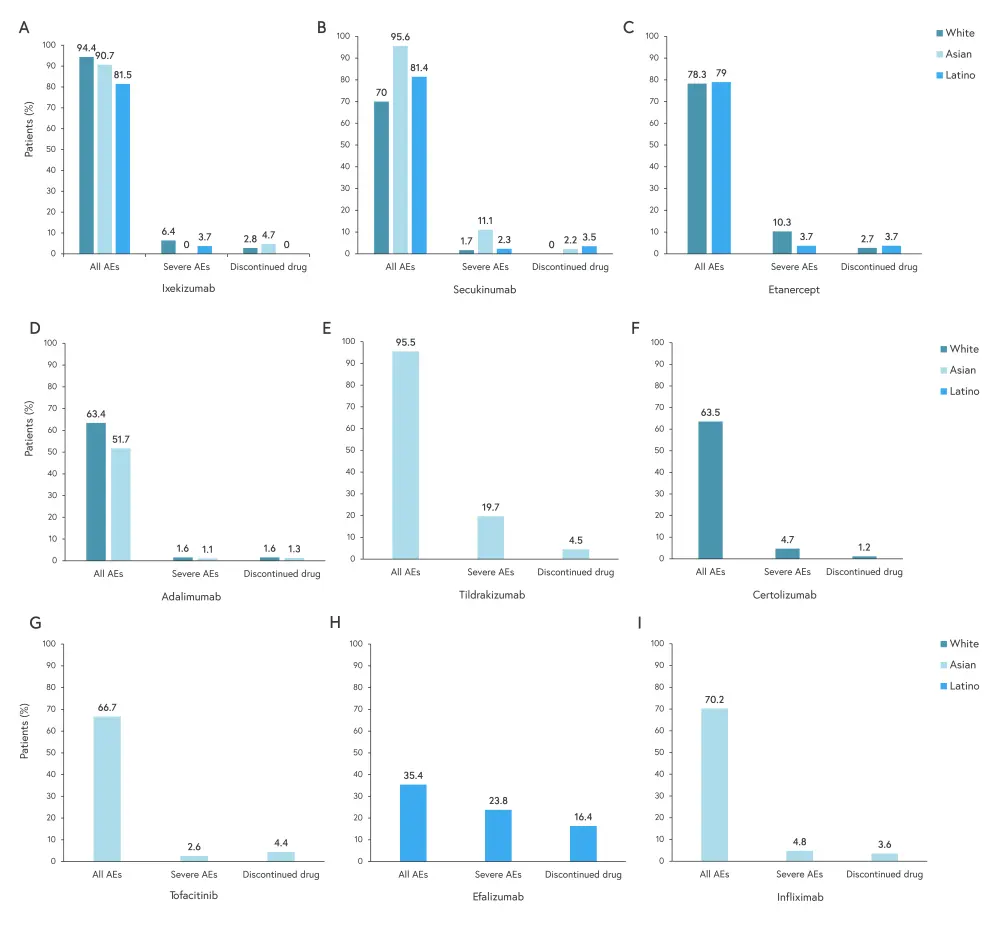
AE, adverse event.
*Adapted from Ferguson, et al.1
There was no AE data reported in any study for black patients. Of all drugs evaluated for AEs,
- Asian patients had the highest rate of AEs and severe AEs when treated with secukinumab;
- White patients had the highest rate of AEs when treated with ixekizumab and severe AEs when treated with etanercept; and
- Latino patients had the highest rate of AEs when treated with ixekizumab and severe AEs when treated with efalizumab.
There was only one death reported in all included studies, an Asian patient who died during a study of adalimumab, which was caused by heart failure possibly related to treatment. The drug with the highest disparity in AE data between race and ethnicity was secukinumab, with Asian patients having the highest incidence of AEs and severe AEs.
Conclusion
Of the treatments included, White patients saw the highest clear/almost clear response to guselkumab, Black patients had the highest response to brodalumab, and Asian and Latino patients had the highest response to ixekizumab. Treatment responses to ustekinumab and secukinumab were very similar across reported races/ethnicities, although more Black patients achieved PASI75 with ustekinumab treatment.
The observed differences in skin clearance and PASI75 depending on race/ethnicity could be due to differences in weight and genetics. The authors theorized that a lower mean weight among Asian patients (mean BMI, 27) led to them receiving a higher concentration of the treatment drug on flat-dosing regimens, resulting in superior skin clearance. Other studies have suggested certain genes being associated with improved efficacy with tumor-necrosis factor inhibitors in European patients.
This review also demonstrated that patients of color are less likely to receive biologics compared with White patients. There may be barriers to healthcare access for Black patients, including a lack of appropriate care and increased healthcare costs, leading to disparities in treatments regimens and overall poorer outcomes. The authors noted several limitations of this study, including a lack of data for some of the races/ethnicities included in certain studies and lack of diversity in enrolled patients into clinical trials; this resulted in a high percentage of White patients in many trials. It was concluded that further studies must aim to include higher numbers of patients of color in psoriasis trials to fully elucidate racial/ethnic differences in safety and efficacy of biologics for psoriasis management.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

