All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
The effect of guselkumab treatment on work productivity and daily activity in patients with PsA
Introduction
Psoriatic arthritis (PsA) can affect the quality of life of patients by impacting their daily lives and ability to work. Here, we discuss results from the phase III DISCOVER-2 trial that assessed the daily activity and work productivity of biologic naïve patients with PsA who were treated with guselkumab for one year, with an additional year of post-hoc analysis.1
Guselkumab is an inhibitor of the p19 subunit of interleukin-23. The study design can be seen in our previous article which presented health-related quality of life outcomes (EQ-5D-5L and EQ-VAS) from the DISCOVER-2 trial. Patients included in DISCOVER-2 received either guselkumab every 4 or 8 weeks or placebo (from Week 24, patients receiving placebo could crossover to receive guselkumab every 4 weeks).1
Baseline work productivity and daily activity1
A total of 738 patients reported daily activity at baseline, with 475 patients employed at baseline and therefore able to report work productivity scores. The majority of patients were white (≥97% across groups), with an average PsA disease duration of 5–6 years. Patients had active disease, with an average of 12–13 swollen joints, 20–22 tender joints, and mean Psoriasis Area and Severity Index (PASI) score of 9.3–10.8. Patients had a mean 36-Item Short Form Health Survey (SF-36) Physical Component Score (PCS) of 32.4–33.3, with an average SF-36 PCS score of 50 reported for the U.S. population as a whole. The work productivity and daily non-work activity at baseline is presented in Figure 1.
Figure 1. Work productivity and daily activity at baseline*
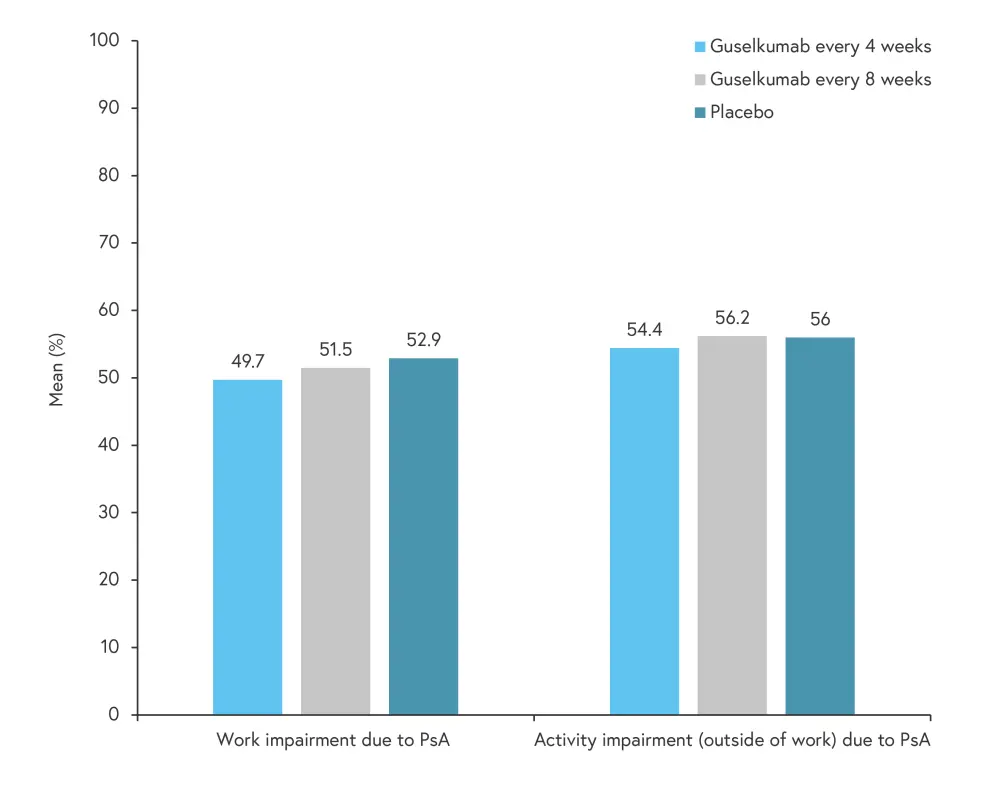
PsA, psoriatic arthritis.
*Adapted from Curtis.1
Work impairment due to PsA can be spilt into absenteeism and presenteeism (Figure 2). Approximately 10% work impairment was missed work due to PsA, which equates to an average of 2 missed days of work per month, whereas 46.9–49.3% of work impairment was due to presenteeism.
Figure 2. Work missed due to PsA*
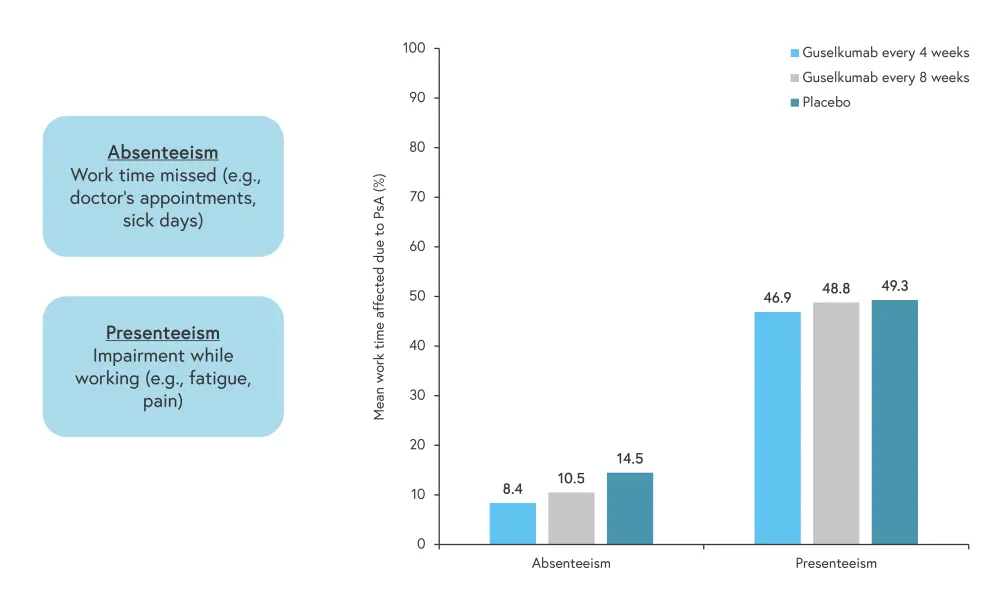
PsA, psoriatic arthritis.
*Adapted from Curtis.1
Impact of guselkumab on work productivity and daily activity1
Improvements were seen in both the work productivity and daily activity of patients treated with guselkumab through to Week 100. The proportion of patients achieving a minimally important difference in daily non-work activity and work productivity is shown in Figure 3.
Figure 3. Proportion of patients achieving MID in A daily activity and B work productivity*
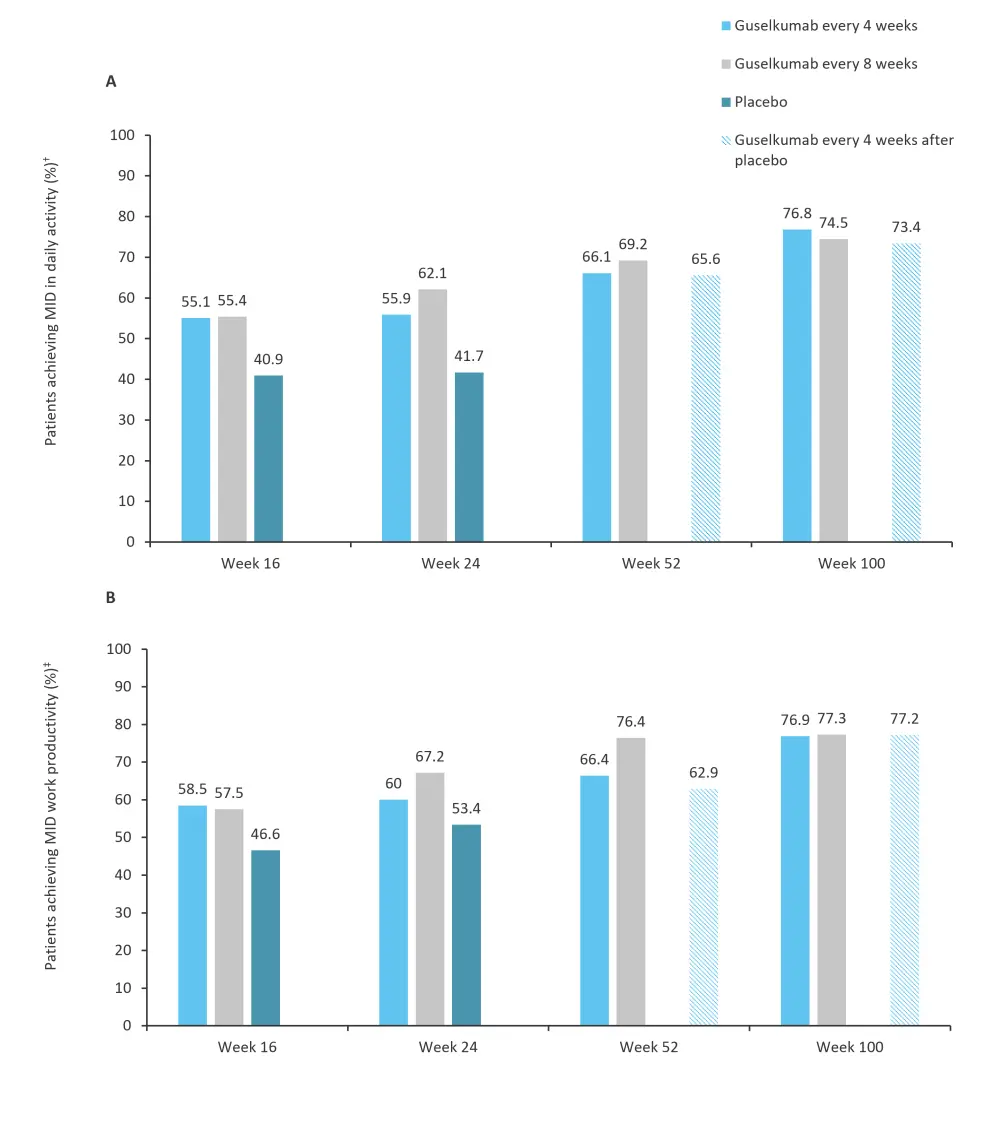
MID, minimally important difference.
*Adapted from Curtis.1
†MID defined as ≥20% improvement among patients with ≥20% impairment at baseline.
‡MID defined as ≥15% improvement among patients with ≥15% work productivity.
Among patients who were employed at baseline, ≥84% remained in employment through to Week 100 regardless of guselkumab dosing regimen. In patients who were not employed at baseline, 20.0–25.3% were employed at Week 100.
Conclusion
This study showed the impact PsA can have on daily life for patients. At baseline, ~50% of patients reported impairments in their daily activity or work productivity due to their PsA; however, both guselkumab dosing regimens allowed the majority patients to achieve minimally important differences in both daily activity and work productivity by Week 100. However, Curtis acknowledged that this cost saving is likely to vary largely when professions and salaries are taken into account. Nevertheless, treatment with guselkumab was shown to improve daily activity and work productivity compared with placebo which may enable more people to gain employment. Gaining and maintaining employment may improve quality of life for these patients, allowing them to live life less affected by psoriatic disease.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

