All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Impact of guselkumab on health-related quality of life for patients with PsA
Introduction
Guselkumab, which inhibits the p19 subunit of interleukin-23, has been evaluated for the treatment of patients with psoriatic arthritis (PsA). The efficacy and safety of guselkumab was analyzed in the phase III DISCOVER-1 (NCT03162796) and DISCOVER-2 (NCT03158285) trials.1,2
The DISCOVER-1 trial met its primary endpoint, ACR20 at Week 24 was achieved by 59% of patients treated every 4 weeks and 52% of those treated every 8 weeks, compared with 22% in the placebo group.1 Similar results were achieved in the phase III DISCOVER -2 trial, with 64% of all patients treated with guselkumab achieving ACR20 response at Week 24 compared with 33% in the placebo group.2 As a result of these studies, guselkumab received approvals from the U.S. Food and Drug Administration (FDA) and the European Commission (EC) in 2020.
In a recent analysis by Curtis et al.,3 the impact of guselkumab on health-related quality of life (HRQoL) was assessed in patients with PsA who had not previously received a biologic.
Study design
DISCOVER-2 was a phase III randomized, double-blind, placebo-controlled trial of adults with active PsA (Figure 1).
Active PsA was defined as
- ≥5 swollen joints (swollen joint count);
- ≥5 tender joints (tender joint count; TJC); and
- a ≥0.6 mg/dL C-reactive protein level despite standard therapy.
Figure 1. Study design*
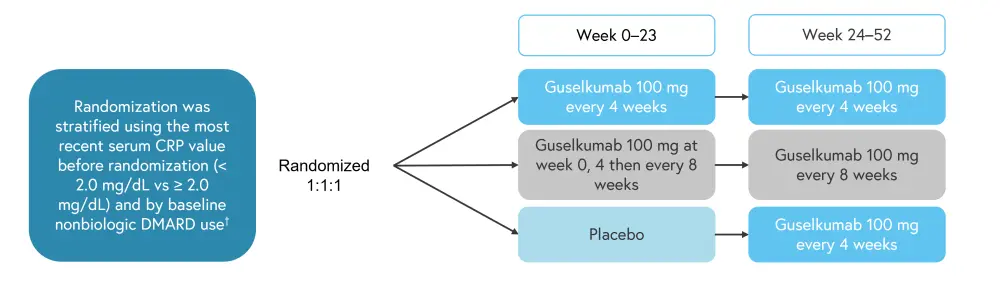
DMARD, disease-modifying antirheumatic drugs.
*Adapted from Curtis, et al.3
†DMARD use options: yes or no.
Two instruments, the EuroQol 5-Dimension 5-Level (EQ-5D-5L) Index and the EuroQol Visual Analog Scale (EQ-VAS), were administered to patients in the DISCOVER-2 trial to assess HRQoL.3
Results
Overall, 739 patients were included in this study with an average age of 45.7 years, 47.5% were female and 98% were White. Patients had a mean swollen joint count (0–66) and tender joint count (0–68) of 12.3 and 21.3, respectively. The average pain score was 6.3/10, with baseline EQ-VAS and EQ-5D-5L scores demonstrating that patients’ HRQoL was greatly impacted at the start of the study. Fatigue was measured using the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), with patients reporting a mean of ~30/52 at baseline.
Table 1. Baseline patient characteristics*
|
BMI, body mass index; CRP, C-reactive protein; EQ-5D-5L, EuroQol 5-Dimension 5-Level; EQ-VAS, EuroQol Visual Analog Scale; FACIT-F, Functional Assessment of Chronic Illness Therapy–Fatigue; HAQ-DI, Health Assessment Questionnaire–Disability Index; IGA Investigator’s Global Assessment, MCS mental component summary, PASI Psoriasis Area and Severity Index, PsA psoriatic arthritis, PCS physical component summary, SD, standard deviation; SF-36, 36-Item Short Form Health Survey; SJC swollen joint count, TJC tender joint count, VAS visual analog scale. |
||||
|
Characteristic |
Guselkumab |
Guselkumab |
Placebo |
All patients |
|---|---|---|---|---|
|
Age, years |
45.9 (11.5) |
44.9 (11.9) |
46.3 (11.7) |
45.7 (11.7) |
|
Female, % |
42.0 |
48.0 |
52.4 |
47.5 |
|
White, % |
98.8 |
96.8 |
98.4 |
98.0 |
|
BMI, kg/m2 |
29.1 (5.9) |
28.7 (6.3) |
29.0 (6.4) |
28.9 (6.2) |
|
PsA disease duration, years |
5.5 (5.9) |
5.1 (5.5) |
5.8 (5.6) |
5.5 (5.7) |
|
SJC, mean (SD) |
12.9 (7.8) |
11.7 (6.8) |
12.3 (6.9) |
12.3 (7.2) |
|
TJC, mean (SD) |
22.4 (13.5) |
19.8 (11.9) |
21.6 (13.1) |
21.3 (12.9) |
|
Patient pain score, mean (SD)† |
6.2 (2.0) |
6.3 (2.0) |
6.3 (1.8) |
6.3 (1.9) |
|
HAQ-DI, mean (SD)† |
1.2 (0.6) |
1.3 (0.6) |
1.3 (0.6)‡ |
1.3 (0.6)§ |
|
Median CRP level, mg/dL |
1.2 |
1.3 |
1.2 |
1.2 |
|
Enthesitis, % |
170 (69.4) |
158 (63.7) |
178 (72.7)‡ |
506 (68.6)§ |
|
Dactylitis, % |
121 (49.4) |
111 (44.8) |
99 (40.4)‡ |
331 (44.9)§ |
|
PASI, mean (SD)† |
10.8 (11.7) |
9.7 (11.7) |
9.3 (9.8)‡ |
9.9 (11.1)§ |
|
IGA psoriasis total score ≥2, % |
201 (82.0) |
195 (78.6) |
209 (85.3)‡ |
605 (82.0)§ |
|
EQ-5D-5L Index, mean (SD)‖ |
0.6 (0.1) |
0.6 (0.2) |
0.6 (0.1)‡ |
0.6 (0.1)§ |
|
EQ-VAS, mean (SD)¶ |
46.9 (20.1) |
44.5 (19.8) |
42.5 (19.2)‡ |
44.6 (19.7)§ |
|
FACIT-F, mean (SD)† |
30.8 (9.6) |
29.3 (9.9) |
29.1 (9.5)‡ |
29.7 (9.7)§ |
|
SF-36 PCS score, mean (SD)# |
33.3 (7.1) |
32.6 (7.9) |
32.4 (7.0)‡ |
32.8 (7.3)§ |
|
SF-36 MCS score, mean (SD)# |
48.4 (11.0) |
47.4 (10.8) |
47.2 (12.0)‡ |
47.7 (11.3)§ |
Patients in the guselkumab group demonstrated an improvement from baseline in EQ-5D-5L Index and EQ-VAS scores after 16 weeks, this improvement was maintained through to Week 52 (Figure 2). Patients treated with guselkumab every 8 weeks showed a significant increase compared with the placebo group at Week 16 in both HRQoL scores (EQ-5D-5L, p = 0.0368; EQ-VAS, p = 0.0015). At Week 24, both guselkumab treated groups showed a significant improvement compared with the placebo group (Figure 2).
After Week 24, patients treated with the placebo were allowed to swap to the guselkumab group treated every 4 weeks. Following this treatment change, patients in the crossover group showed an improvement in HRQoL in line with patients originally randomized to receive guselkumab every 4 weeks (66.1 vs 66.2% in EQ-5D-5L and 68.1% vs 67.1% in the EQ-VAS crossover vs guselkumab group treated every 4 weeks, respectively).
Figure 2. Proportion of patients who achieved improvements ≥minimally important difference in A EQ-5D-5L score and B EQ-VAS through to Week 52*
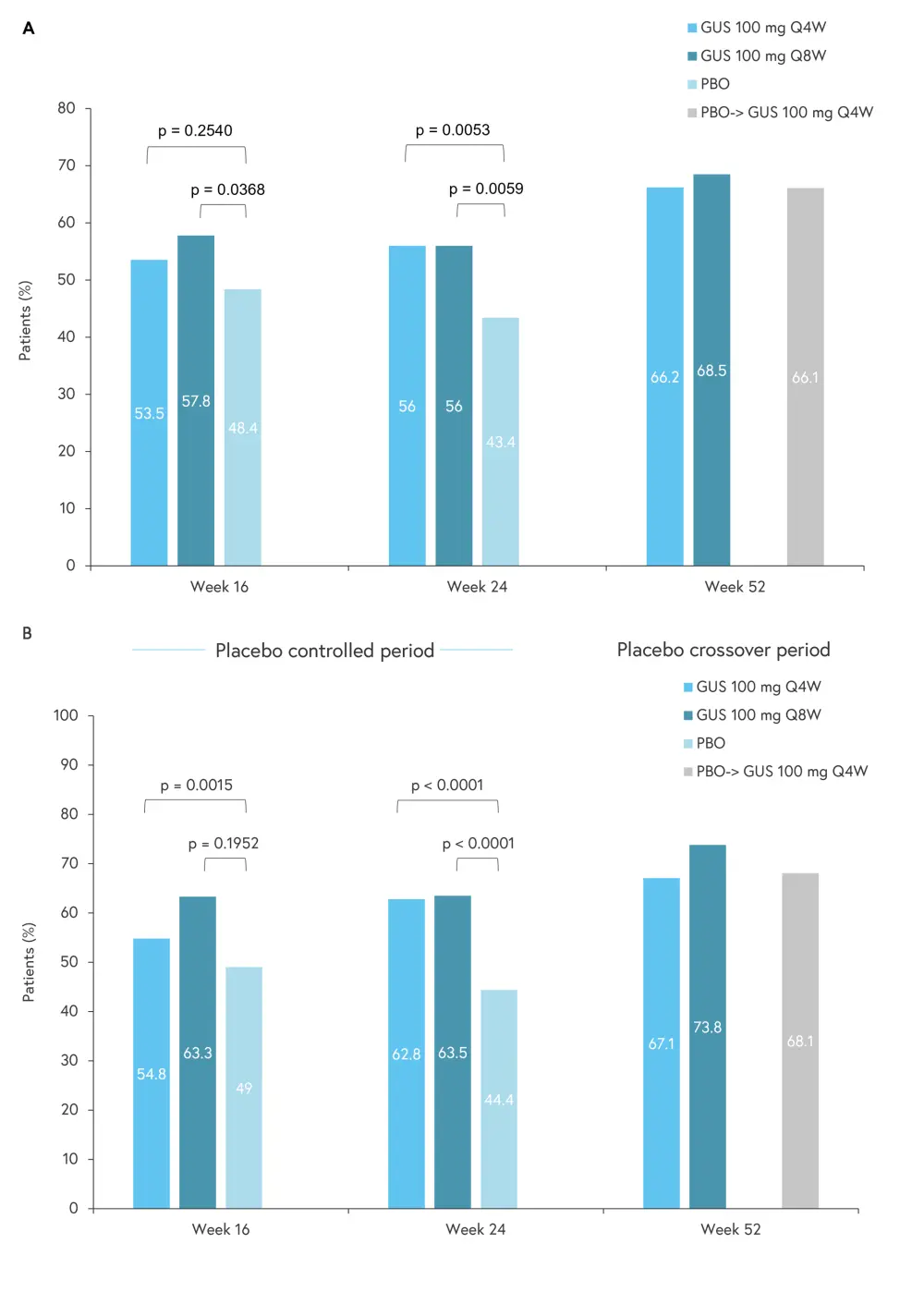
EQ-5D-5L, EuroQol 5-Dimension 5-Level; EQ-VAS, EuroQol Visual Analog Scale; GUS, guselkumab, PBO, placebo; Q4W, every 4 weeks; Q8W, every 8 weeks.
*Adapted from Curtis, et al.3
†The minimally imporant difference values were 0.07 and 9.68 for EQ-5D-5L and EQ-VAS, respectively.
Multivariate analysis was conducted to assess what clinical features were associated with HRQoL; parameters demonstrating a significant association are detailed in Table 2. FACIT-F and pain scores were significantly associated with both the EQ-5D-5L index and EQ-VAS scores.
Table 2. Multivariate analysis of patient variables with EQ-5D-5L Index and EQ-VAS from baseline to Week 24*
|
CRP, C-reactive protein; EQ-5D-5L, EuroQol 5-Dimension 5-Level; EQ-VAS, EuroQol Visual Analog Scale; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; MMRM, mixed-effects models for repeated measures; PASI, Psoriasis Area and Severity Index; SJC, swollen joint count; TJC, tender joint count. |
||||
|
Parameter |
EQ-5D-5L Index |
EQ-VAS |
||
|---|---|---|---|---|
|
Estimate |
p value |
Estimate |
p value |
|
|
CRP, mg/dL |
-0.005 |
<0.0001 |
-0.51 |
0.007 |
|
FACIT-F† |
0.007 |
<0.0001 |
0.57 |
<0.0001 |
|
Pain‡ |
-0.02 |
<0.0001 |
-3.47 |
<0.0001 |
|
PASI§ |
-0.0005 |
0.03 |
-0.17 |
0.0001 |
|
SJC‖ |
-0.0005 |
0.21 |
-0.17 |
0.02 |
|
TJC¶ |
-0.0005 |
0.04 |
-0.04 |
0.41 |
|
Dactylitis |
0.01 |
0.02 |
1.74 |
0.49 |
Limitations
The authors noted several limitations of this study, including the impact of specific inclusion criteria in DISCOVER-2 on the generalizability of the findings. The EQ-5D-5L score was translated to an index score between 0−1, with a statistical mapping program that used the US EuroQoL value set; therefore, using values from a different country may give a slightly different result.
Conclusion
Patients treated with 100 mg guselkumab every 4 or 8 weeks in the DISCOVER-2 trial demonstrated an improvement in both HRQoL scoring systems, with benefit seen up to Week 52 of analysis. HRQoL impairment for patients with PsA was associated with C-reactive protein level, fatigue, pain, increased psoriasis symptoms, and swollen/tender joints. Guselkumab treatment resulted in improved clinical symptoms and led to an improvement of HRQoL in patients with PsA.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

