All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
The DERMIS trials of roflumilast for chronic plaque psoriasis
DERMIS-1 and DERMIS-2 are identically designed randomized, double-blind, controlled, multicenter phase III trials investigating the safety and efficacy of roflumilast cream for patients with chronic plaque psoriasis.1 Roflumilast, a potent phosphodiesterase 4 inhibitor, is currently used orally to manage chronic obstructive pulmonary disease and is now being investigated as topical treatment for several dermatologic conditions.
Previous trials of topical roflumilast in psoriasis reported long-term efficacy with no new safety signals observed. Here, we discuss the results published by Lebwohl et al., including IGA success and other efficacy and safety outcomes of the DERMIS trials.1
Study design1
Patients aged 2 years or older with a clinical diagnosis of plaque psoriasis were randomized 2:1 to receive either 0.3% roflumilast cream once daily, or vehicle cream, for 8 weeks. Randomization was performed according to a computer-generated randomization list, with treatment blinded to patients, investigators, and the sponsor. Patients were instructed to apply roflumilast cream to all areas of psoriasis identified at baseline, including palms and sole if affected; however, these areas were not evaluated for efficacy. The primary efficacy endpoint was Investigator Global Assessment (IGA) success at Week 8 (achieving a clear or almost clear IGA status plus a ≥2 grade improvement from baseline), evaluated on a scale of 0 (clear) to 4 (severe). Treatment-emergent adverse events (TEAEs) and serious adverse events of roflumilast treatment vs vehicle cream were evaluated in the safety analysis. The trial design is shown in Figure 1 and baseline patient characteristics are shown in Table 1.
Figure 1. Trial design*
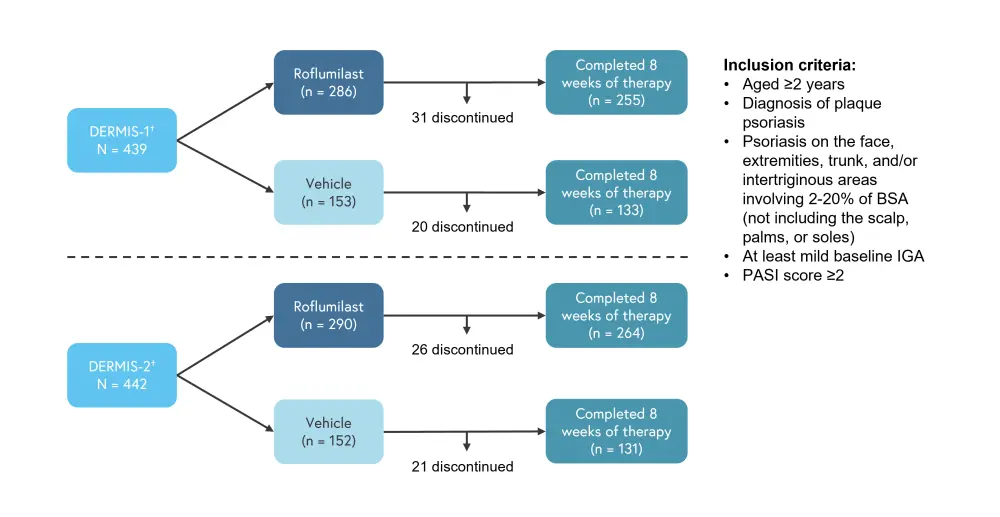
BSA, body surface area; IGA, Investigator Global Assessment; PASI, Psoriasis Area and Severity Index.
†Randomization was stratified by study site, baseline IGA score (2 vs 3), and intertriginous involvement at baseline (I-IGA score <2 vs ≥2).
*Adapted from Lebwohl, et al.1
Table 1. Baseline patient characteristics*
|
BMI, body mass index; BSA, body surface area; IGA, Investigator Global Assessment; I-IGA, intertriginous-IGA; PASI, Psoriasis Area and Severity Index. |
|||||
|
Characteristic, % |
DERMIS-1 trial |
DERMIS-2 trial |
|
||
|---|---|---|---|---|---|
|
Roflumilast |
Vehicle |
Roflumilast |
Vehicle |
|
|
|
Mean age, years |
47.6 |
48.7 |
46.9 |
47.1 |
|
|
Sex |
|
|
|
|
|
|
Female |
33.9 |
37.3 |
39.3 |
34.2 |
|
|
Male |
66.1 |
62.7 |
60.7 |
65.8 |
|
|
Mean BMI |
32.0 |
31.9 |
31.0 |
33.5 |
|
|
Race |
|
|
|
|
|
|
American |
1.4 |
0.7 |
0 |
0.7 |
|
|
Asian |
7.3 |
7.2 |
6.9 |
5.9 |
|
|
Black or |
2.8 |
5.2 |
4.5 |
5.9 |
|
|
Native |
0.7 |
0 |
1.0 |
0.7 |
|
|
White |
81.8 |
81.0 |
82.8 |
82.9 |
|
|
Not reported |
1.4 |
2.0 |
1.7 |
1.3 |
|
|
Other |
3.8 |
3.3 |
2.8 |
2.6 |
|
|
More than |
0.7 |
0.7 |
0.3 |
0 |
|
|
Mean psoriasis-affected BSA |
6.3 |
7.4 |
7.1 |
7.7 |
|
|
Mean IGA score |
2.9 |
2.9 |
2.9 |
2.9 |
|
|
Mean PASI score |
5.7 |
5.7 |
5.8 |
6.1 |
|
|
I-IGA score ≥2 |
22.0 |
20.9 |
18.3 |
20.4 |
|
|
Psoriasis area of involvement |
|
|
|
|
|
|
Face |
25.9 |
29.4 |
26.2 |
25.7 |
|
|
Genitalia |
17.8 |
13.7 |
15.9 |
12.5 |
|
|
Intertriginous |
23.8 |
21.6 |
19.3 |
21.1 |
|
Results1
Primary efficacy outcome
IGA success outcomes in DERMIS-1 and DERMIS-2 trials are shown in Figure 2. At Week 8 from baseline, IGA success was significantly higher in patients treated with roflumilast compared with the vehicle cream in both trials.
Figure 2. IGA success in patients treated with roflumilast compared with vehicle*
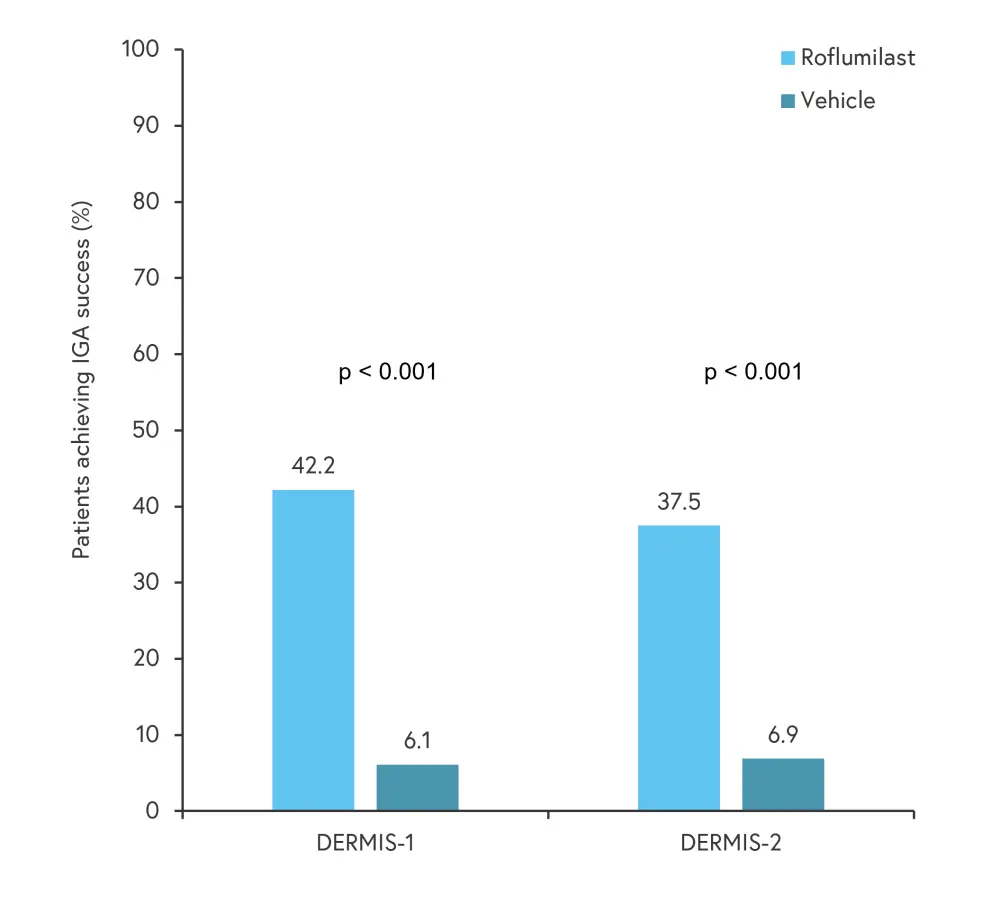
IGA, Investigator Global Assessment.
*Adapted from Lebwohl, et al.1
Other efficacy outcomes
IGA success was also measured at Week 4, with a significant improvement seen in patients treated with roflumilast compared with the vehicle (DERMIS-1, 20.6% vs 2.3%; p < 0.001; DERMIS-2, 19.1% vs 5.3%; p < 0.001). In patients with an intertriginous-IGA score of ≥2 at baseline, treatments with roflumilast significantly increased the percentage of patients achieving IGA success at Week 8 compared with vehicle (DERMIS-1, 71.2% vs 13.8%; p < 0.001; DERMIS-2, 68.1% vs 18.5%; p < 0.001). In addition, the percentage of patients achieving a 75% and 90% reduction in Psoriasis Area and Severity Index scores from baseline was significantly increased in patients treated with roflumilast, as shown in Figure 3.
Figure 3. Week-8 A PASI 75 and B PASI 90*
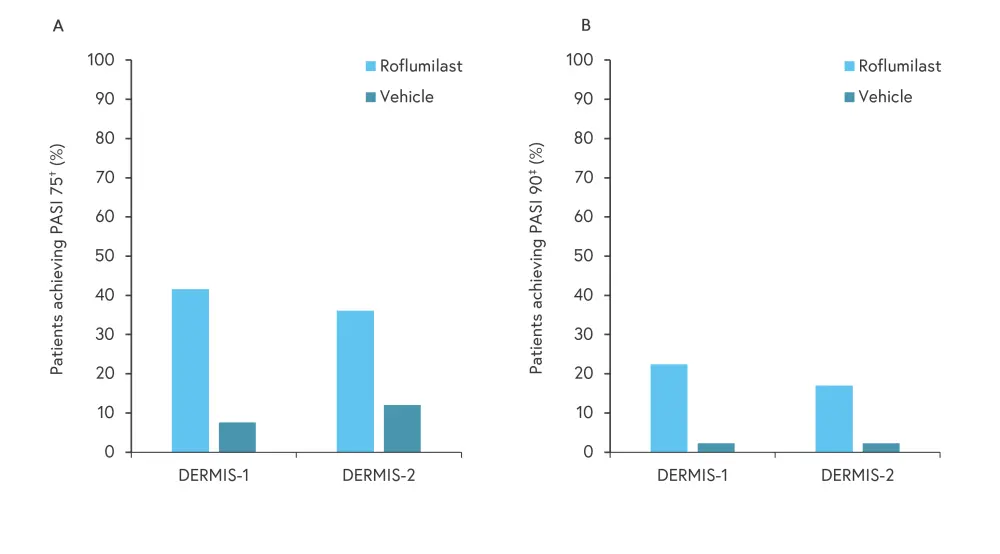
PASI, Psoriasis Area and Severity Index.
†75% reduction in PASI score.
‡90% reduction in PASI score.
*Adapted from Lebwohl, et al.1
Safety
- In DERMIS-1, 25.2% of patients treated with roflumilast and 23.5% of patients treated with vehicle experienced a TEAE; in both cohorts, 0.7% were serious adverse events.
- In DERMIS-2, 25.9% of patients treated with roflumilast and 18.4% of patients treated with vehicle experienced any TEAE. Overall, 0% and 0.7% of patients treated with roflumilast and vehicle experienced a serious adverse event, respectively.
- Less than 2% of patients across the roflumilast treatment groups in both trials discontinued due to adverse events.
The most common TEAEs are shown in Figure 4.
Figure 4. Most TEAEs (≥2% in any treatment group)*
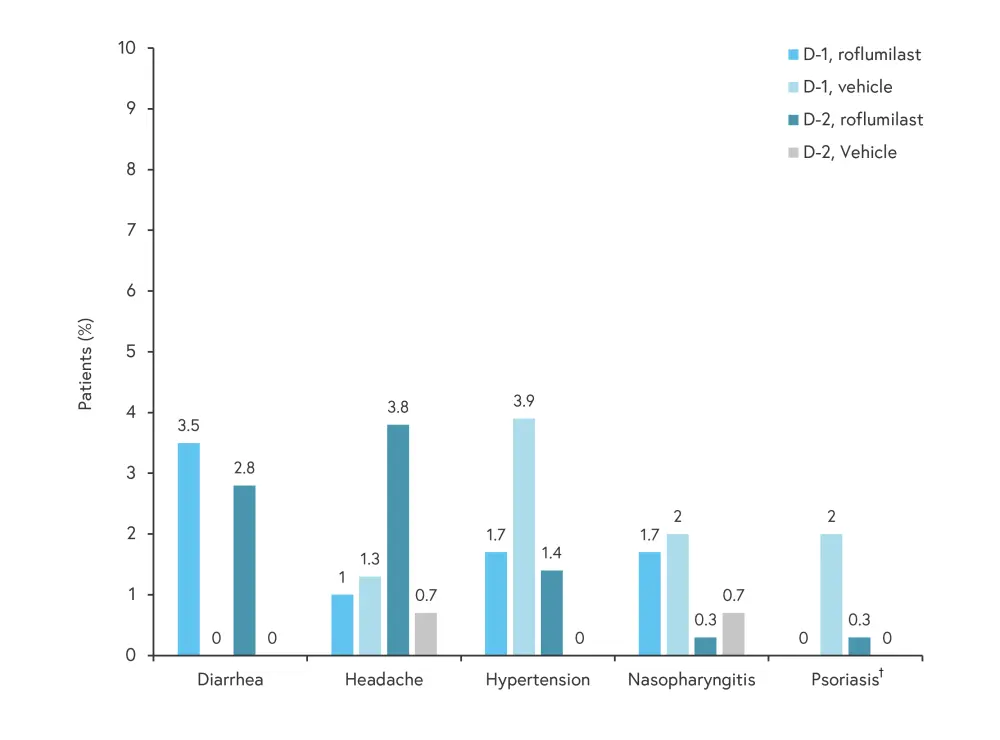
D-1, DERMIS-1; D-2, DERMIS-2; TEAE, treatment-emergent adverse event.
†Progression of psoriasis that was out of proportion to the natural history of psoriasis.
*Adapted from Lebwohl, et al.1
Conclusion
In both DERMIS-1 and DERMIS-2, roflumilast cream showed significant efficacy at 8 weeks from baseline in patients with chronic plaque psoriasis compared with the vehicle control. IGA success at Week 8 was improved, with significant improvements seen in intertriginous-IGA status. In roflumilast and vehicle treatment cohorts, similar occurrence of TEAEs was observed across both trials, with an overall low occurrence of serious adverse events.
The authors noted several limitations of these studies, such as the inability to make direct comparisons between roflumilast and other active treatments due to the lack of a comparator treatment group. Also, safety and efficacy were only evaluated at the 8-week follow-up; therefore, additional studies are required to determine the long-term safety and efficacy of roflumilast.
Despite these limitations, roflumilast appears to be an effective topical option for patients with chronic plaque psoriasis. The American Academy of Dermatology acknowledge that most patients with plaque psoriasis may benefit from a topical treatment option. Current long-term treatment with topical corticosteroids has been associated with adverse events and limitations when used in intertriginous areas. Therefore, roflumilast may be an alternative treatment option for these patients, especially considering the significant improvements shown in intertriginous-IGA success status.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

