All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
The COMFORT trial: piclidenoson versus apremilast for plaque psoriasis
Piclidenoson is a highly selective small molecule targeting the A3 adenosine receptor (A3AR) that is overexpressed in keratinocytes in patients with psoriasis, inhibiting interleukin (IL)-17 and IL-23 production. A3AR has low or no expression in normal cells.1 Piclidenoson has shown promising anti-psoriatic effects in early clinical proof-of-concept phase II/III trials in moderate to severe plaque psoriasis.1
At the European Academy of Dermatology and Venereology Congress 2022, Papp reported safety and efficacy results at Week 32 from the proof-of-concept phase III, randomized COMFORT trial of piclidenoson in patients with plaque psoriasis.
Study design2
The double-blind, randomized, placebo-controlled COMFORT trial included patients with moderate to severe plaque psoriasis randomized 3:3:3:2 to twice-daily treatment with either piclidenoson 2 mg, piclidenoson 3 mg, apremilast 30 mg, or placebo, with the placebo cohort re-randomized (1:1:1) to treatment after Week 16. The study duration was 32 weeks with an optional extension of up to 48 weeks. The trial design is shown in Figure 1.
Figure 1. Trial design*
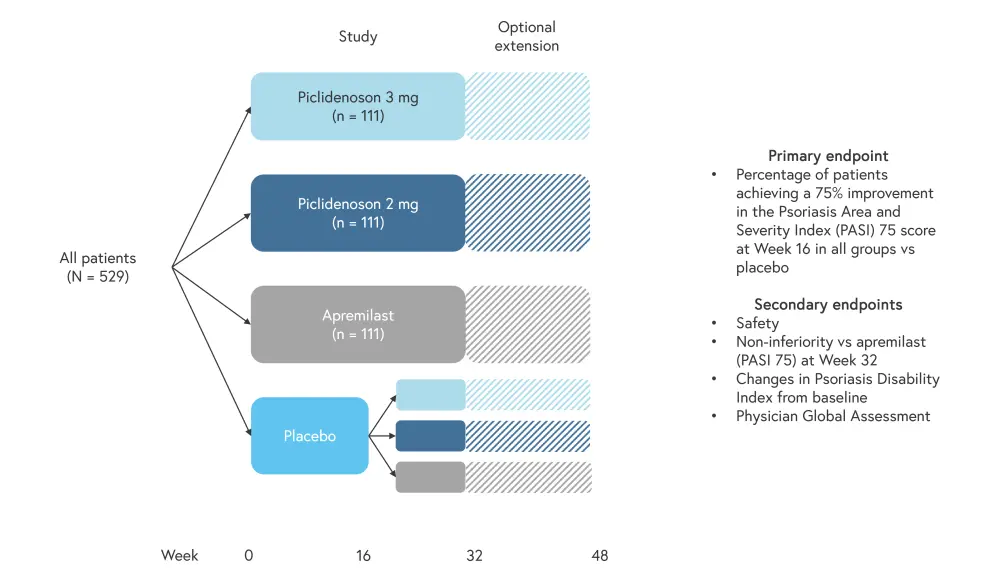
*Adapted from Papp et al.2
Results
Safety1
Patients treated with piclidenoson exhibited fewer adverse events (AEs) than those treated with aprelimast, with a similar safety profile to the placebo group (Table 1). Of note was the difference in gastrointestinal (0.7% vs 6.3% for the piclidenoson 3 mg vs apremilast group, respectively) and nervous system disorder (0.7% vs 9.9% for the piclidenoson 3 mg vs apremilast group, respectively) AEs. In addition, the apremilast group had a higher discontinuation rate than the piclidenoson group.
Table 1. Occurrence of adverse events
|
*Data from Papp.1 |
|||
|
Adverse event, % |
Piclidenoson 3 mg |
Apremilast |
Placebo |
|---|---|---|---|
|
Any |
14.8 |
27.5 |
25.5 |
|
Gastrointentinal disorders |
0.7 |
6.3 |
0 |
|
Infections/infestations |
5.6 |
6.3 |
7.4 |
|
Metabolism/nutrition |
0.7 |
2.1 |
1.1 |
|
Nervous system |
0.7 |
9.9 |
3.2 |
|
Skin and subcutaneous |
2.1 |
2.8 |
6.4 |
Efficacy
Efficacy data included a total of 426 patients (piclidenoson 2 mg, n = 127; piclidenoson 3 mg, n = 103; apremilast 30 mg, n = 118; placebo, n = 78).2 Piclidenoson 3 mg was superior to placebo at Week 16 (Figure 2a) but inferior to apremilast at Week 32 (Figure 2b).2 However, patients treated with piclidenoson had comparable improvement in Psoriasis Disability Index from baseline to Week 32 compared with those treated with apremilast.1 Additionally, response to piclidenoson was seen to increase over time for both Psoriasis Area and Severity Index 75 and Physician Global Assessment scores up to Week 32.1
Figure 2. PASI 75 score at week 16 and week 32*
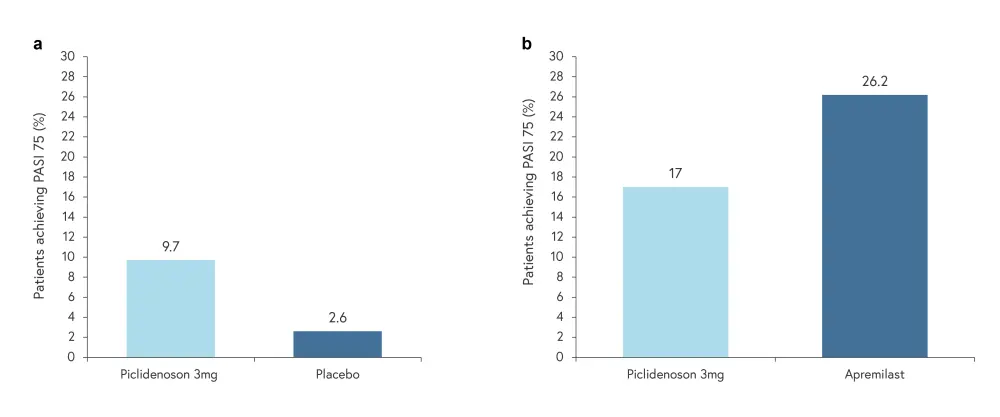
PASI, Psoriasis Area and Severity Index.
*Adapted from Papp, et al.2
Conclusion
These findings demonstrate the superior efficacy of piclidenoson compared with placebo with statistically significant improvement in PASI 75 at week 16; however, it was shown to be comparable in efficacy to apremilast in patients with plaque psoriasis. The safety profile was acceptable, with fewer adverse events reported than those treated with apremilast and a significant reduction in gastrointestinal and nervous system disorder AEs.1 The authors concluded that these results support the continued clinical development of piclidenoson, with a pivotal phase III trial is currently being planned.2
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

