All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Do you know... Which of the following disproportionately impacts patients with psoriasis and skin of color and can have a significant impact on quality of life?
On February 6, 2025, the PsOPsA Hub held a Clinical Trial Club webinar on improving outcomes for people of color with chronic plaque psoriasis including scalp involvement.
Here, we share the presentation by Andrew Alexis, Weill Cornell Medical College, New York, US, and Mona Shahriari, Yale University, New Haven, US, which explored the study design (Figure 1), unique approach, and safety and efficacy outcomes from the VISIBLE trial in people of color.
Spotlight on the VISIBLE trial in people of color
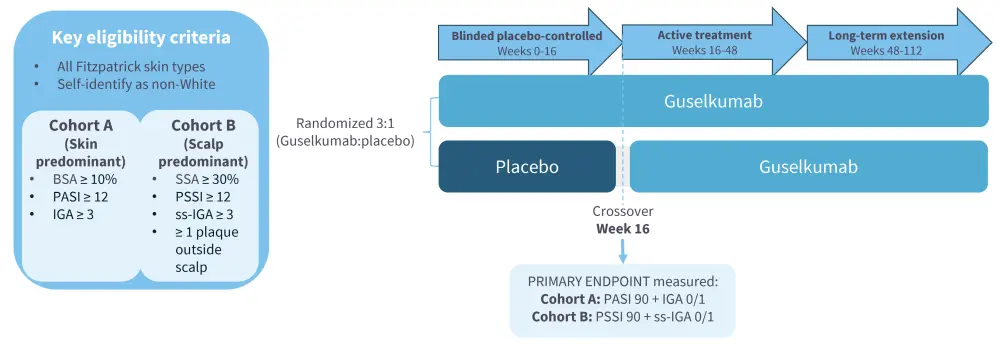
Figure 1. Study design of VISIBLE (NCT05272150)*
Key takeaways
- The VISIBLE trial aimed to address gaps that have previously occurred in psoriasis clinical trials, such as the exclusion of some groups (e.g., Middle Eastern with traditional race ethnicity categories), difficultly in differentiating active disease from post-inflammatory pigment, and the subjectiveness of Fitzpatrick skin type.
- Therefore, VISIBLE employed novel approaches to increase diversity of skin types and racial/ethnic groups in a psoriasis trial, including1:
- Use of colorimetry to determine skin tone within Fitzpatrick skin type framework (I–VI)
- Inclusion of self-identified non-White racial/ethnic categories
- Enhanced photography with centralized review
- Reducing barriers to enrollment and retention
- Novel exploratory endpoints
- In total, 211 patients were enrolled and randomized in the trial; co-primary endpoints were2:
- Proportion of participants achieving an Investigator’s Global Assessment (IGA) score of cleared (0) or minimal (1), and proportion of participants achieving a Psoriasis Area and Severity Index (PASI) 90 response at Week 16, in Cohort A (skin predominant)
- Proportion of participants achieving a scalp-specific IGA (ss-IGA) score of absence of disease (0) or very mild disease (1), and proportion of participants achieving a Psoriasis Scalp Severity Index (PSSI) 90 response at Week 16, in Cohort B (scalp predominant)
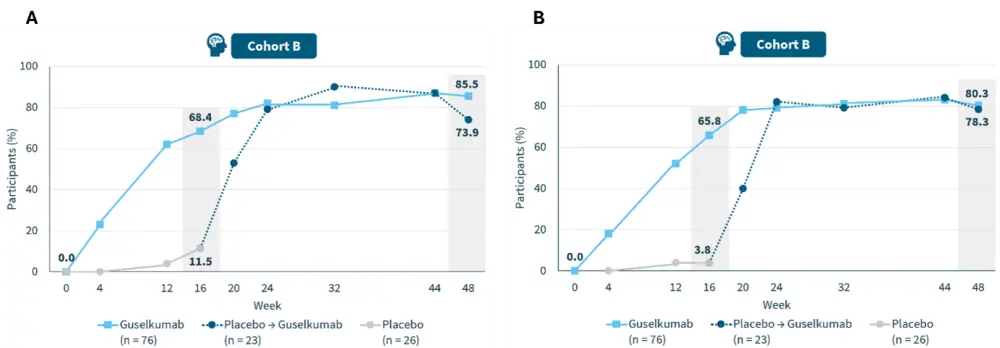
- Significant skin (Figure 2) and scalp clearance (Figure 3) was demonstrated with guselkumab across diverse skin tones, meeting co-primary and secondary endpoints through Week 48.3
- Patients reported rapid improvements in quality of life metrics, including Dermatology Life Quality Index (DLQI), and reductions in skin discoloration, which can disproportionally affect people of color, starting as early as Week 16.4
Figure 2. Proportion of patients achieving A PASI 90 and B IGA 0/1 through Week 48*
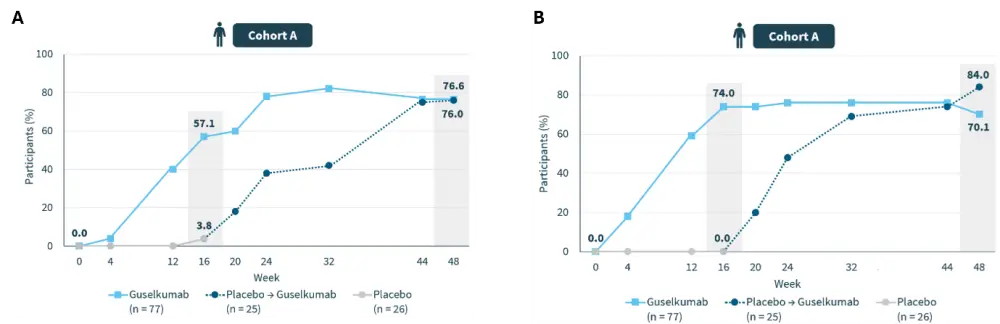
Figure 3. Proportion of patients achieving A ss-IGA 0/1 and B PSSI 90 through Week 48*
The VISIBLE trial in people of color | Panel discussion
This independent educational activity was supported by Janssen Biotech, Inc., administered by Janssen Scientific Affairs. All content was developed independently by the faculty. The funder was allowed no influence on the content of this activity.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?


 Mona Shahriari
Mona Shahriari Andrew Alexis
Andrew Alexis