All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
KEEPsAKE 1 and KEEPsAKE 2: Efficacy and safety of risankizumab in PsA
Introduction
Psoriatic arthritis (PsA) can be debilitating for patients and can cause arthritis, enthesitis, and dactylitis. Risankizumab, a monoclonal antibody that inhibits interleukin-23, is approved for the treatment of active PsA.1 At the European Academy of Dermatology and Venereology 2022 Congress, Papp presented updated results from the phase III KEEPsAKE 1 and KEEPsAKE 2 trials of risankizumab for PsA.1 The Psoriasis and Psoriatic Arthritis Hub has previously reported on the Week-24 efficacy and safety results of the KEEPsAKE 1 trial.
Study design1
Both KEEPsAKE 1 (NCT03675308) and KEEPsAKE 2 (NCT03671148) are double-blind, placebo-controlled trials investigating the safety and efficacy of risankizumab in adults with active PsA who had inadequate response/intolerance to ≥1 conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs); KEEPsAKE 2 also included those who had inadequate response/intolerance one or two biologic therapies. The study design for both studies is illustrated in Figure 1. Patients were randomized 1:1 to risankizumab or placebo, with doses given at baseline, 4, and 16 in Period 1. At Week 24, patients randomized to placebo in Period 1 received a blinded dose of RZB and patients randomized to RZB received a blinded dose of placebo, maintaining blinding of treatment in Period 1. At Week 24, the primary endpoint of ≥20% improvement in American College of Rheumatology score (ACR20) was evaluated. At Week 28, patients randomized to placebo in Period 1 received a second dose of RZB and patients randomized to RZB received a scheduled RZB dose; all patients received RZB every 12 weeks thereafter.
Figure 1. Study design*
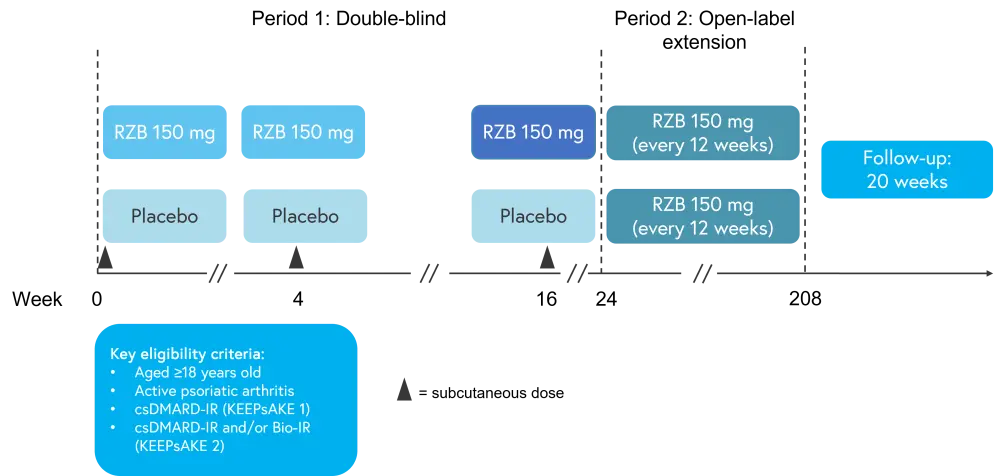
Bio-IR, biologic therapy; csDMARD-IR, conventional synthetic disease-modifying antirheumatic drug; RZB, risankizumab.
*Adapted from Papp.1
Results1
All efficacy and safety results relate to patients originally randomized to risankizumab and percentages discussed are based on as observed values. In both KEEPsAKE 1 and 2, the percentage of patients achieving ACR20 improved to Week 40 and was maintained to Week 100, as shown in Figures 2 and 3.
Figure 2. Percentage of patients achieving ACR20 in KEEPsAKE 1*
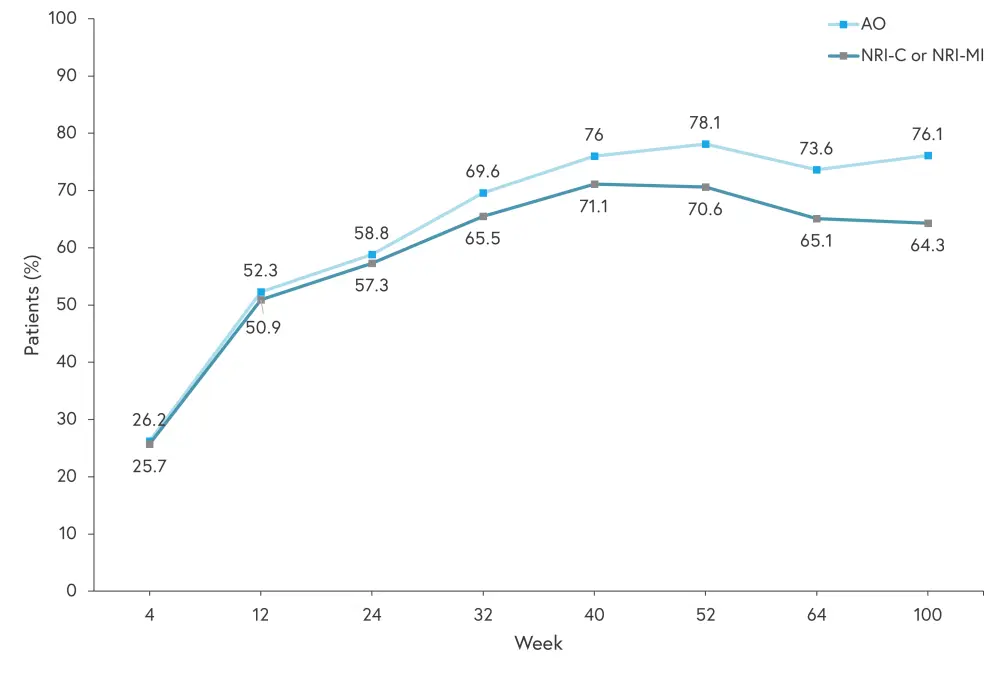
AO, as observed; NRI-C, non-responder imputation incorporating multiple imputation to handle missing data due to COVID-19; NRI-MI, as observed with missing data imputed as non-responders except those missing due to COVID-19 or geo-political conflict in Ukraine and Russia, which are imputed by multiple imputation.
*Adapted from Papp.1
Figure 3. Patients achieving ACR20 in KEEPsAKE 2*
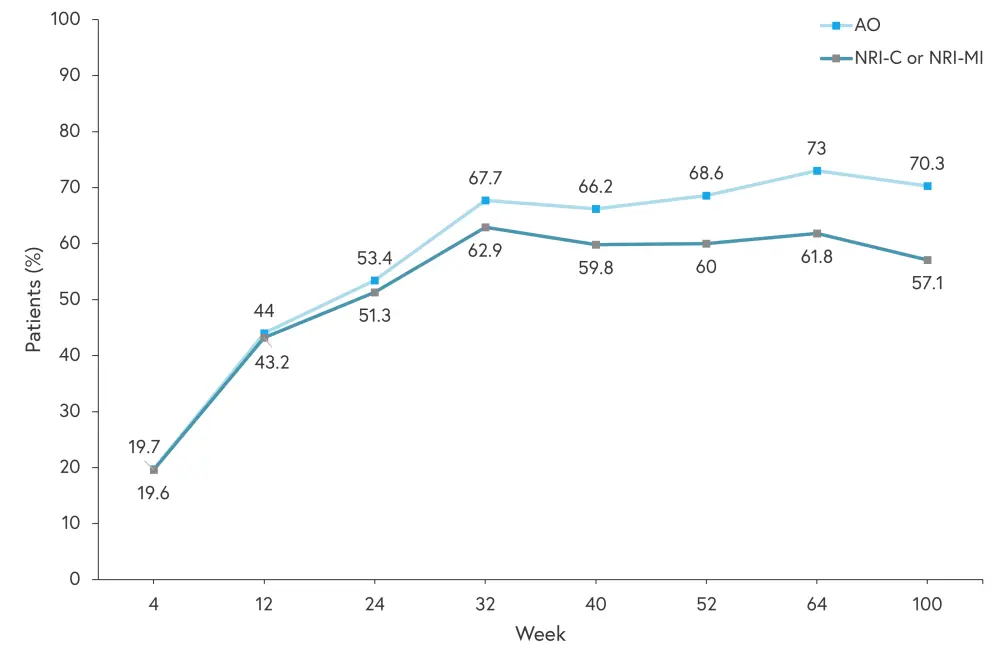
AO, as observed; NRI-C, non-responder imputation incorporating multiple imputation to handle missing data due to COVID-19; NRI-MI, as observed with missing data imputed as non-responders except those missing due to COVID-19 or geo-political conflict in Ukraine and Russia, which are imputed by multiple imputation.
*Adapted from Papp.1
Similarly, ACR50 and ACR70 (50% and 70% improvement in American College of Rheumatology score) also improved between Week 24 and Week 40 and was maintained to Week 100 in both trials. ACR50 improved from 33.3% to 50% and from 27.3% to 42.9% at Week 24 and Week 100 in KEEPsAKE 1 and KEEPsAKE 2, respectively. ACR70 improved from 15% to 31.6% and from 12.3% to 26.2% in KEEPsAKE 1 and KEEPsAKE 2, respectively. The percentage of patients achieving minimal disease activity at baseline, Week 24, and Week 100 is shown in Figure 4.
Figure 4. Minimal disease activity*
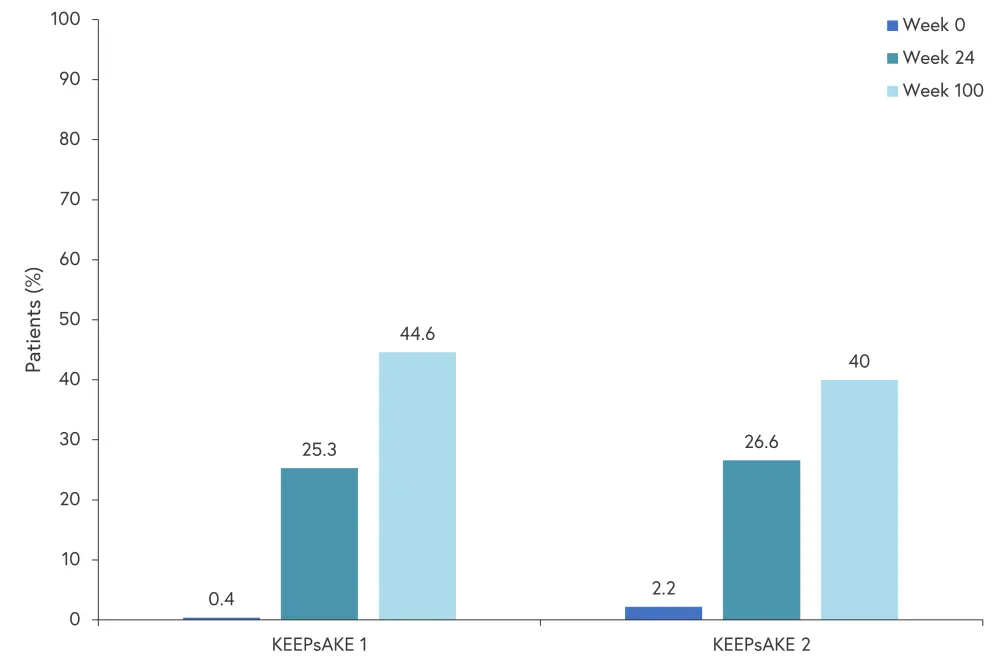
*Adapted from Papp.1
Additionally, Papp presented key efficacy data supporting long-term durable improvement after risankizumab treatment (Table 1). Combined data across KEEPsAKE 1 and 2 demonstrated 71.1% and 91.6% of patients experiencing a resolution of enthesitis and dactylitis, respectively, by Week 100.
Table 1. Week-100 efficacy results of key additional efficacy endpoints. Changes are least square mean changes from baseline*
|
AO, as observed; FACIT, functional assessment of chronic illness therapy; HAQ-DI, health assessment questionnaire-disability index; MRMM, mixed-effect model repeated measures; NRI-MI, non-response imputation by multiple imputation; PASI 90, ≥90% reduction in psoriasis area severity index; SF-36 PCS; 36 item short form health survey physical component summary. |
||||
|
Outcome |
KEEPsAKE 1 (n = 483) |
KEEPsAKE 2 (n = 224) |
||
|---|---|---|---|---|
|
AO |
NRI-MI or |
AO |
NRI-MI or |
|
|
Change in HAQ-DI, mean |
−0.42 |
−0.41 |
−0.30 |
−0.26 |
|
PASI90 (n/N)‡, % |
81.4 (192/236) |
71.3 (195/273) |
79.8 (83/104) |
67.5 (83/123) |
|
Change in SF-36 PCS, mean |
8.6 |
8.4 |
7.0 |
6.42 |
|
Change in FACIT-Fatigue, mean |
7.9 |
7.8 |
34.4 |
5.4 |
Key safety data was also presented (Table 2), highlighting no new safety signals since Week 24. A total of eight patients died across KEEPsAKE 1 and 2. Causes of death included acute leukemia complications; septicemia, resulting from anastomosis surgery for diverticulitis; pneumonia, resulting in urosepsis; cardiorespiratory arrest 166 days after last dose; coronary artery plaque rupture; death from an unknown cause (subject had been hospitalized for anxiety and depression, developed septicemia after discharge, and died one week later); and COVID-19 related complications.
Table 2. Week-100 safety results*
|
MACE, major adverse cardiovascular events; PBO, placebo; RZB, risankizumab; TEAE, treatment-emergent adverse event. |
||||
|
TEAEs |
KEEPsAKE 1 |
KEEPsAKE 2 |
||
|---|---|---|---|---|
|
RZB Week 24 |
RZB and PBO/RZB |
RZB Week 24 |
RZB and PBO/RZB |
|
|
Any TEAE |
398 |
2,223 |
286 |
1,462 |
|
Serious TEAE |
15 |
130 |
14 |
80 |
|
TEAE leading to discontinuation |
6 |
36 |
2 |
10 |
|
COVID-19 related TEAE |
1 |
137 |
1 |
72 |
|
Any MACE |
0 |
2 |
1 |
4 |
|
Any serious infection |
6 |
39 |
3 |
13 |
|
Any serious hypersensitivity |
0 |
1 |
0 |
1 |
|
Deaths |
1 |
7 |
0 |
1 |
Conclusion
The updated Week-100 results further demonstrate the long-term durable efficacy of risankizumab in patients with PsA. Papp highlighted that risankizumab was generally well tolerated, with no new or unanticipated safety signals at Week 100. This data shows that risankizumab may be an option for patients in whom csDMARDs and/or biologic agents are ineffective or not tolerated.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

