All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
The safety and frequency of adverse events with spesolimab treatment
Introduction
Dysregulation of the IL-36 pathway has been implicated in several inflammatory diseases, such as generalized pustular psoriasis (GPP), palmoplantar pustulosis, and atopic dermatitis. Spesolimab, a first-in-class anti-interleukin (IL)-36R antibody, has been shown to be efficacious and safe for the management of GGP flares.
Spesolimab was recently granted Food and Drug Administration (FDA) approval for the treatment of GPP flares, as previously reported on the Psoriasis and Psoriatic Arthritis Hub. At the 2022 European Academy of Dermatology and Venereology Congress (EADV), Mockenhaupt presented safety data from five clinical trials investigating spesolimab.1
Study design1
The five clinical trials include a phase I trial (NCT02978690) and four phase II, double-blind, randomized placebo-controlled trials (NCT03782792; NCT03135548; NCT04015518; NCT03822832). A detailed summary of the studies is shown in Table 1. Across the five studies, 222 patients were treated with spesolimab and 100 patients received placebo.
Table 1. Study details*
|
AD, atopic dermatitis; GPP, generalized pustular psoriasis; N/A, not applicable; PPP, palmoplantar pustulosis. |
|||||
|
Study characteristics |
Study 1 |
Study 2 |
Study 3 |
Study 4 |
Study 5 |
|---|---|---|---|---|---|
|
Disease type |
GPP |
GPP |
PPP |
PPP |
AD |
|
Phase |
I |
II |
IIa |
IIb |
IIa |
|
Spesolimab |
10 mg/kg |
900 mg |
900 mg or |
300 mg or |
600 mg every |
|
Administration |
Intravenous |
Intravenous |
Intravenous |
Subcutaneous |
Intravenous |
|
Total patients, |
7 |
53 |
59 |
152 |
51 |
|
Spesolimab |
7 |
35 |
38 |
109 |
33 |
|
Placebo |
N/A |
18 |
21 |
43 |
18 |
Safety1
The rate ratio of adverse events (AEs) for spesolimab versus placebo was 1.3, 0.9, 0.9, and 1.2 for studies 2, 3, 4, and 5, respectively. Adverse event occurrence, including severe and serious AEs, is shown in Figure 1; there were no patient deaths reported across the five studies. Infections were mostly mild and uncomplicated, the most common are outlined in Figure 2. Two of the placebo-controlled trials demonstrated a lower infection rate in the spesolimab group (Trial 3 and Trial 4), while two showed a lower infection rate in the placebo group (Trial 2 and Trial 5). Upper respiratory tract infections were most common but others, such as urinary tract infections, were also reported.
Figure 1. Incidence of adverse events*
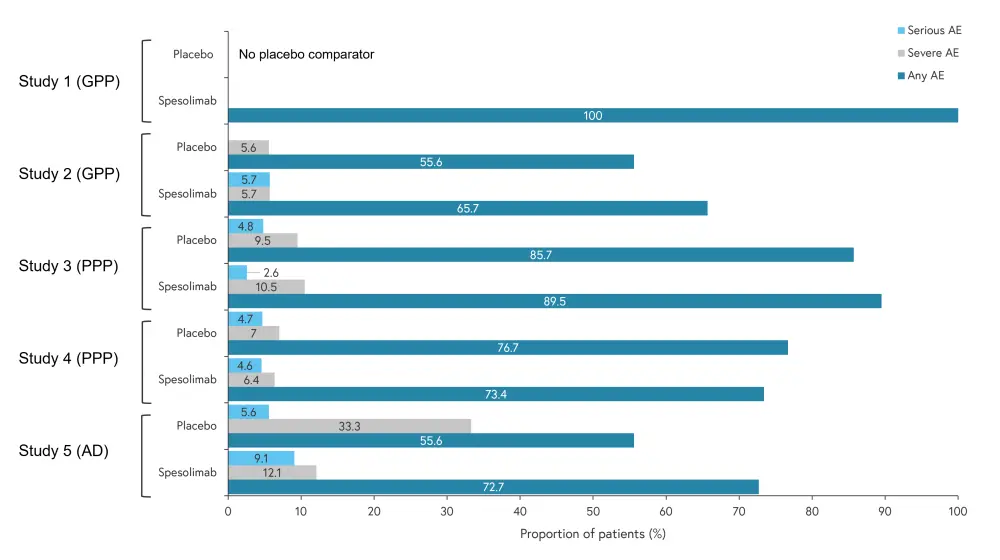
AE, adverse event.
*Adapted from Mockenhaupt.1
†Rheumatology Common Toxicity Criteria ≥3.
‡A patient may have serious adverse events with multiple seriousness criteria.
Figure 2. Overall infection rate and most common infection per study*
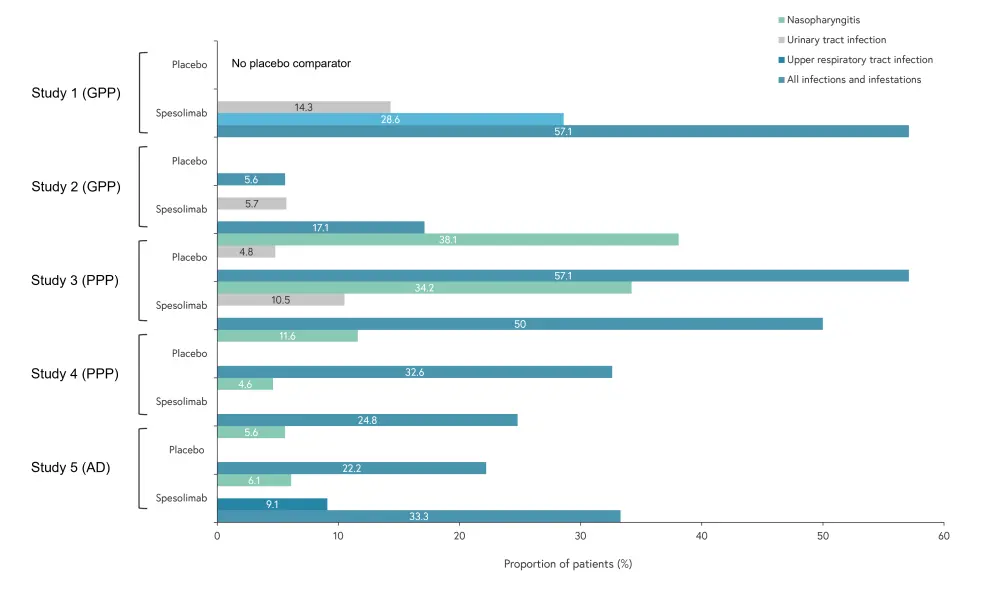
*Adapted from Mockenhaupt.1
Rates of hypersensitivity (eczema, rash, allergic rhinitis, and drug reaction with eosinophilia and systemic symptoms), anaphylactic reactions (including circulatory collapse), and angioedema (eye oedema and urticoria) were evaluated in Studies 2, 4, and 5. There was a similar frequency of hypersensitivity between the placebo and spesolimab groups, with the highest frequency observed in Study 5 (33.3% and 44.4% in the spesolimab and placebo arms, respectively). Eosinophil values were also measured for all five trials, there was no evidence of eosinophilia associated with spesolimab treatment. High eosinophils (>1 × 109/L) were observed in two patients (28.6%) in Study 1 and four patients in the placebo (28.6%) and four patients in the spesolimab (12.9%) arm of Study 5. For all other trials, no patients in either arm were found to have a high eosinophil count. Although elevation in liver enzymes was recorded, this was not found to be associated with spesolimab treatment.
Conclusion1
Analysis of these five clinical trials showed a similar frequency of AEs between the placebo and treatment arms. Spesolimab was shown to have a favorable safety profile, with no evidence of treatment-associated eosinophilia in patients with GPP, palmoplantar pustulosis, or atopic dermatitis; however, this was in a limited number of patients. This data and its recent FDA approval suggest spesolimab could prove an effective treatment option for patients with GPP and other chronic inflammatory diseases.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

