All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Safety of biologic/methotrexate combination therapy vs biologic monotherapy in psoriasis
In moderate-to-severe cases of psoriasis, combinations of biologic drugs and conventional systemic drugs (e.g., methotrexate) are often prescribed.1 However, there is a lack of safety data available for these combinations.1
This study by Lluch-Galcerá et al.,1 published in British Journal of Dermatology, aimed to assess whether using certain combinations of biologic drugs with methotrexate increased the incidence of adverse events (AEs), compared with biologic monotherapy. The study used data from the BIOBADADERM registry, which focuses on studying drug safety.1
Study design and patient population1
- Of the 4,486 patients in the BIOBADADERM registry (2008–2021), this study analyzed 2,829 patients, who underwent 5,441 treatment cycles (monotherapy [90.5%], combination [9.5%]). Overall, this equated to 12,853 patient-years.
- Across the treatment groups, most patients were male, had a diagnosis of plaque psoriasis, and had prior biologic treatments.
- The primary outcome was the adjusted incidence rate ratios for total AEs, serious AEs (SAEs), and AEs by system organ class for combination vs monotherapy.
- Biologics investigated in this study were:
-
- Tumor necrosis factor (TNF) inhibitors (etanercept, infliximab, or adalimumab and their biosimilars);
- Interleukin-23 inhibitors (ustekinumab, tildrakizumab, guselkumab, or risankizumab); and
- IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab).
Key findings1
- A total of 7,230 AEs were reported, with the majority in patients receiving biologic monotherapy (the predominant treatment group) (Figure 1A).
- Overall, patients on combined therapy had a higher incidence of AEs, but this was not significant. The incidence of SAEs was similar between the groups (Figure 1B).
- A significant increase in gastrointestinal AEs was seen in patients receiving TNF inhibitors plus methotrexate compared with TNF inhibitor therapy alone (adjusted incidence rate ratio 2.50; 95% confidence interval, 1.57–3.98). This significant difference was not seen for any other combinations.
Figure 1. A) Division (%) of AEs between treatment groups, B) Incidence of AEs and SAEs per 1,000 person-years for each treatment group*
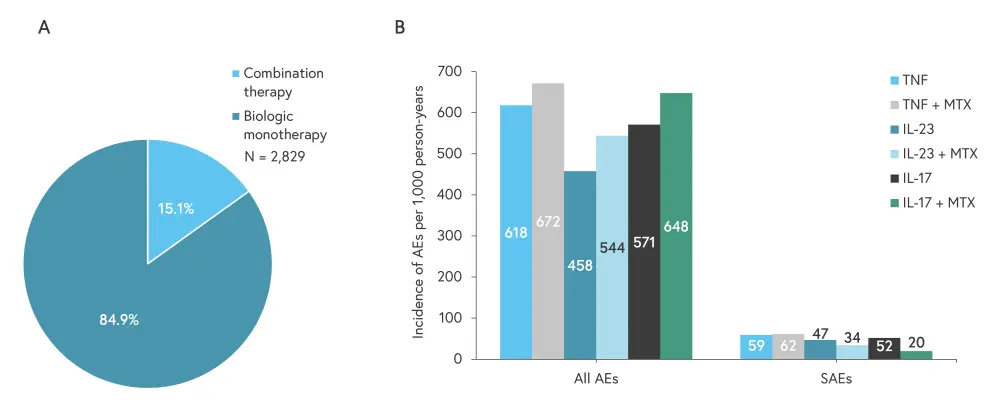
AE, adverse event; IL, interleukin; MTX, methotrexate; SAE, serious adverse event; TNF, tumor necrosis factor.
*Data from Lluch-Galcerá, et al.1
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

