All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Real-world study of upadacitinib efficacy and safety in patients with PsA: UPJOINT trial
Do you know... In the real-world study by Werner et al., what proportion of patients achieved minimal disease activity at Week 24?
Psoriatic arthritis (PsA) is an inflammatory disease that can present with multiple clinical manifestations affecting the joints, spine, tendons/entheses, skin, nails, and other parts of the musculoskeletal system.1 Upadacitinib is a Janus kinase inhibitor approved for use in patients with PsA with an inadequate response to non-biological or biological disease-modifying antirheumatic drugs.
The Psoriasis and Psoriatic Arthritis Hub has previously reported studies assessing the efficacy and safety of upadacitinib; however, real-world data remain limited. Recently, Werner et al.1 published an observational study of upadacitinib in a clinical setting in Rheumatology and Therapy, using data from the UPJOINT trial (NCT04758117). We summarize the key findings below.
Methods1
This was an interim analysis comprising data from the prospective, multicenter UPJOINT trial of upadacitinib over 48 weeks. Eligibility criteria for the UPJOINT trial included age ≥18 years, a diagnosis of active PsA, swollen joint count of ≥1, and undergoing upadacitinib treatment.
Data from the UPJOINT trial was collected from baseline to Week 24; the primary endpoint of the trial was the proportion of patients attaining minimal disease activity (MDA) after 24 weeks of treatment.
In this real-world study, composite and clinical measures included:
- MDA;
- very low disease activity;
- remission or low disease activity according to the Disease Activity Index for PsA;
- tender joint count 68/swollen count joint 66;
- body surface area affected by psoriasis;
- Leeds Enthesitis Index; and
- presence of dactylitis and nail psoriasis.
Patient-reported outcomes included:
- Bath Ankylosing Disease Activity Index;
- Health Assessment Questionnaire-Disability Index;
- Dermatology Life Quality Index;
- Numerical rating scale assessing pain; and
- Numerical rating scale assessing Global Disease Activity.
Results
Baseline characteristics
A total of 296 patients completed the baseline visit, of which 192 also completed the Week 24 visit. The average age at baseline was 54.1 years, 65.5% of patients were female, and 60.5% of patients had oligo- or polyarticular PsA. Table 1 shows the baseline characteristics of patients included in the efficacy analysis.
Table 1. Baseline characteristics of patients included in the efficacy analysis*
|
Characteristics |
Total |
|---|---|
|
Disease duration, years |
8.7 (8.9) |
|
BMI (SD), mean |
29.4 (6.3) |
|
DAPSA (SD), mean |
29.2 (15.0) |
|
SJC66 (SD), mean |
6.0 (6.0) |
|
TJC68 (SD), mean |
9.8 (9.0) |
|
NRS pain (0–10; SD), mean |
6.5 (2.1) |
|
NRS PtGA (0–10; SD), mean |
5.9 (2.5) |
|
BASDAI (SD), mean |
5.3 (2.2) |
|
HAQ-DI (SD), mean |
1.2 (0.7) |
|
DLQI (SD), mean |
6.7 (6.7) |
|
MDA, % |
2.7 |
|
VLDA, % |
0 |
|
BSA, % |
6.1 |
|
Enthesitis, % |
39.2 |
|
Dactylitis, % |
15.2 |
|
Nail psoriasis, % |
29.1 |
|
Previous csDMARDs/GC, % |
88.2 |
|
Pre-therapy with bDMARDS/tsDMARDS, % |
74.3 |
|
Previous oral JAKi, % |
6.8 |
|
B, biological; BASDAI, Bath Ankylosing Disease Activity Index; BMI, body mass index; BSA, body surface area; cs, conventional synthetic; DMARD, disease-modifying antirheumatic drugs; DAPSA, Disease Activity Index for Psoriatic Arthritis; DLQI, Dermatology Life Quality Index; GC, glucocorticoid; HAQ-DI, Health Assessment Questionnaire-Disability Index; JAKi, Janus kinase inhibitor; MDA, minimal disease activity; NRS, numerical rating scale; PtGA, Patient Global Assessment of Disease Activity; ts, targeted-synthetic; TJC68/SJC66, tender joint count/swollen joint count including 68/66 joints; VLDA, very low disease activity. |
|
Efficacy
Composite and clinical measures
The proportion of patients achieving MDA increased from 2.7% at baseline to 39.1% at Week 24 (95% confidence interval [CI], 2.1–46.3). Figure 1 shows the improvement in composite and clinical measures from baseline.
- Of the patients with dactylitis and enthesitis at baseline, 55.2% and 45.1% achieved resolution, respectively.
- A decrease from 19.3% at baseline to 8.9% at Week 24 was reported in patients with a body surface area of >3%.
- Additionally, there was a steady decrease in Disease Activity Index for PsA, with a mean change from baseline to Week 24 of −14.7 (95% CI, −16.4 to −13.0).
Figure 1. Improvement in A composite and B clinical measures from baseline to Week 24*
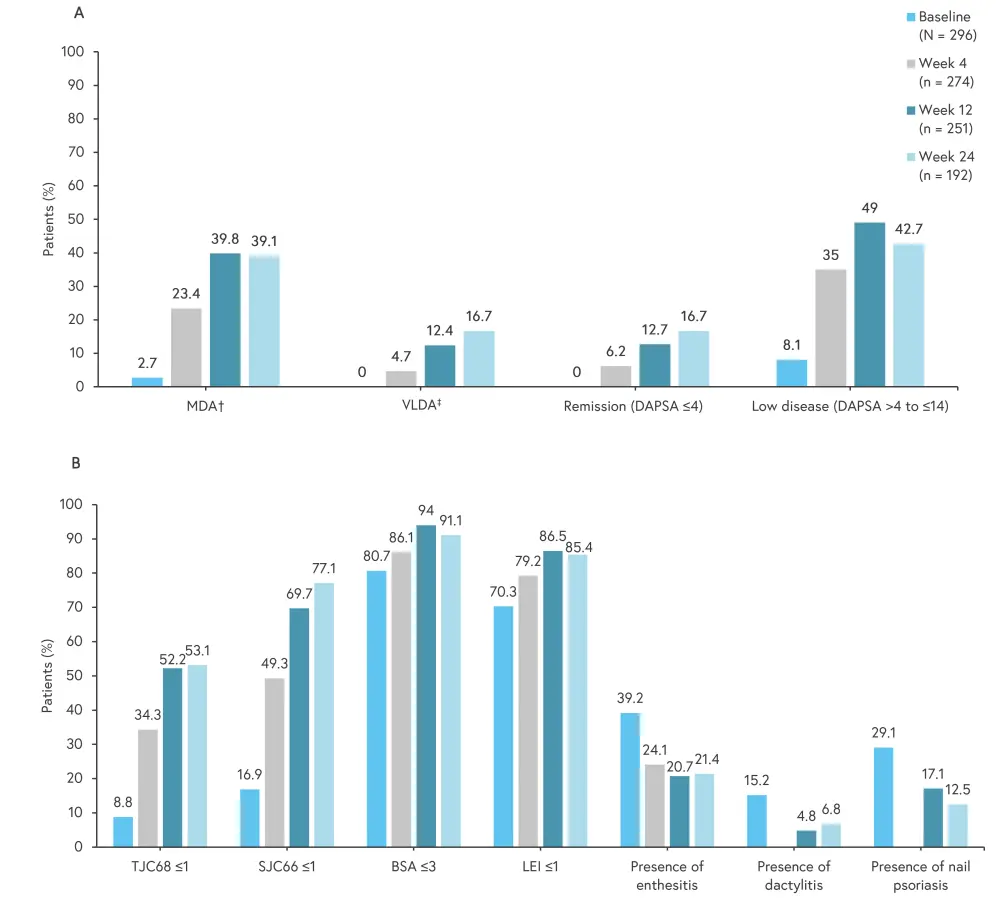
BSA, body surface area; DAPSA, Disease Activity Index for Psoriatic Arthritis; HAQ-DI, Health Assessment Questionnaire Disability Index; LEI, Leeds Enthesitis Index; MDA, minimal disease activity; PASI, Psoriasis Area and Severity Index; PtGA, patient global disease activity; TJC68/SJC66, tender joint count/swollen joint count including 68/66 joints; VAS, visual analog scale; VLDA, very low disease activity.
*Data from Werner, et al.1
†Five of the following seven criteria are fulfilled: tender joint count ≤1; swollen joint count ≤1; PASI ≤1 or BSA ≤3%; patient pain VAS (0–100) ≤15; PtGA (VAS 0–100) ≤20; HAQ-DI ≤0.5; tender entheseal points ≤1.
‡Very low disease activity: All seven criteria mentioned above need to be fulfilled.
Patient-reported outcomes measures
Upadacitinib demonstrated improvement in patient-reported outcomes for disease activity and physical function (Figure 2). The initial BASDAI showed an improvement, with a mean of 5.3 and a standard deviation (SD) of 2.2 at baseline vs 3.6 (SD, 2.4) by Week 24. The mean Dermatology Life Quality Index of 6.7 (SD, 6.7) at baseline decreased to 4.3 (SD, 5.5) by Week 24. Sick leaves were reported by 6.8% vs 10.5% of patients at Week 24 and baseline, respectively.
Figure 2. Patient-reported outcomes measures*
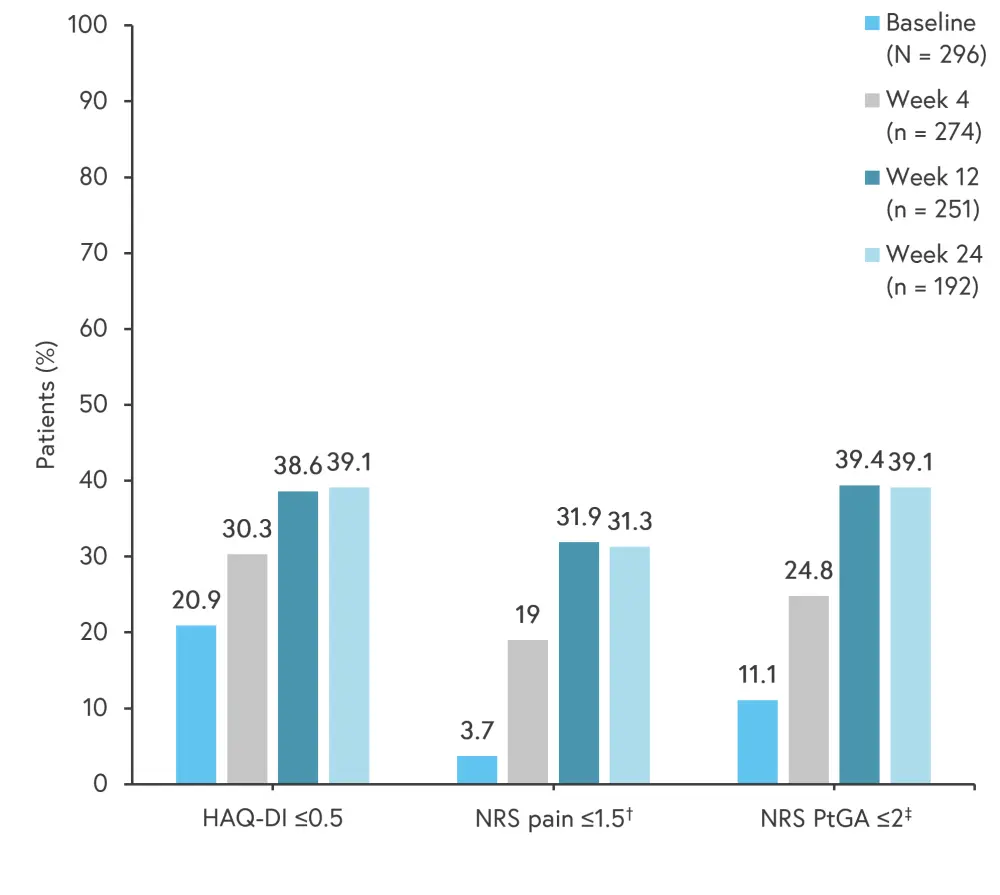
HAQ-DI, Health Assessment Questionnaire-Disability Index; NRS, numerical rating scale; PtGA, Patient’s Global Assessment of Disease Activity.
*Data from Werner, et al.1
†Patient’s assessment of pain was measured using a 0–10 NRS with a cutoff ≤2, given NRS was measured increments of 1 and lacks a distinct value for 1.5. ‡PtGA was measured using a 0–10 NRS.
Safety
Overall, 42% of patients reported adverse events (AEs) of any grade and 4.3% reported serious AEs. Treatment discontinuation due to AEs was reported by 15% of patients. Figure 3 shows the most common AEs of special interest.
Figure 3. Adverse events of special interest*

*Data from Werner, et al.1
Conclusion1
This real-world study demonstrated upadacitinib as an effective in the treatment of active PsA, without any new safety signals. The results confirm previous findings from randomized controlled trials of upadacitinib, indicating its effectiveness in patients with inadequate response to conventional synthetic or biological disease-modifying antirheumatic drugs. Limitations of the study include the use of inferential statistical methods, lack of data on the duration of psoriasis, and follow-up information of patients who left the UPJOINT trial. In addition, the impact of concomitant antirheumatic therapies also needs to be investigated.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?


