All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Orismilast for moderate-to-severe psoriasis: Results from the phase IIb IASOS trial
The phosphodiesterase-4 (PDE-4) enzyme regulates cyclic adenosine monophosphate signaling, involved in the inflammatory cascades responsible for causing psoriasis.1-3 Orismilast is a novel, potent PDE-4 inhibitor with high selectivity to B and D isoforms which are overexpressed in the skin of patients with psoriasis.1 Compared with apremilast, orismilast is at least 2–5 times more potent on all PDE-4 isoforms and up to 39 times more potent on some PDE4-B/D isoforms.1
Orismilast displays a broad-spectrum anti-inflammatory effect by inhibiting the production of tumor necrosis factor (TNF)-α and secretion of T-helper (Th)-1 (TNF-α and interferon [IFN]-γ), Th17 (interleukin [IL]-22 and IL-23), and Th2 (IL-4, IL-5, and IL-13) effector cytokines in preclinical models.2 The efficacy and safety of four different formulations of orismilast and immediate-release tablets were demonstrated in phase I (NCT03812198) and in phase IIa (NCT02888236) trials, respectively, in patients with moderate-to-severe psoriasis.3
At the 2023 American Academy of Dermatology (AAD) Annual Meeting, French presented results of phase IIb IASOS study (NCT05190419) evaluating the efficacy and safety of modified-release orismilast tablets versus placebo in adults with moderate-to-severe psoriasis.1 Here, we summarize the key findings.
Study design1
In this phase IIb, double-blind, placebo-controlled, parallel-group dose-ranging study, eligible patients were randomized 1:1:1:1 to receive one of three active doses of orismilast or placebo twice daily. The study design is illustrated in Figure 1.
Figure 1. Study design, eligibility criteria, and endpoints*
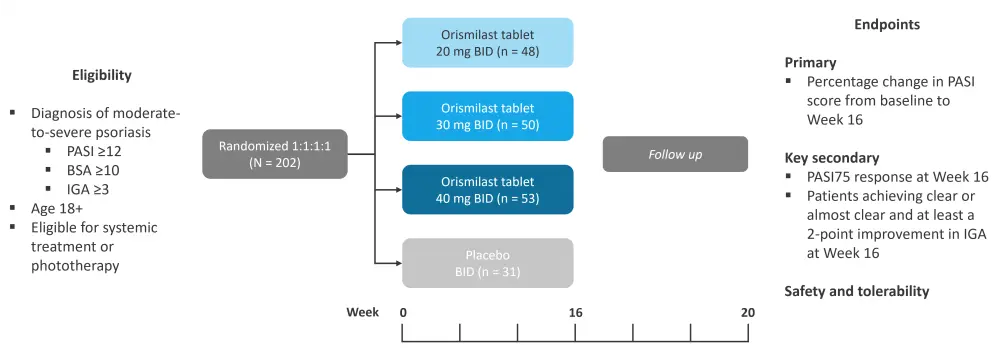
BID, twice daily; BSA, body surface area; IGA, Investigators Global Assessment; PASI, Psoriasis Area and Severity Index.
*Adapted from Warren, et al.1
Results1
A total of 202 patients with moderate-to-severe psoriasis were included (Table 1). The median age was 43.5 years and the majority of patients were male (72.8%) and white (89.1%). Severe psoriasis was predominant in the active arms, whereas moderate psoriasis was predominant in the placebo arm.
Table 1. Baseline patient characteristics*
|
BID, twice daily; BMI, body mass index; BSA, body surface area; DLQI, Dermatology Life Quality Index; IGA, Investigator’s Global Assessment; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis. |
||||
|
Characteristic, % (unless |
Placebo |
20 mg BID |
30 mg BID |
40 mg BID |
|---|---|---|---|---|
|
Median age, years |
42.0 |
42.5 |
47.0 |
44.0 |
|
Male |
76.5 |
64.6 |
78.0 |
71.7 |
|
Median BMI, kg/m2 |
27.7 |
28.8 |
29.5 |
28.8 |
|
White |
86.3 |
89.6 |
92.0 |
88.7 |
|
Median disease duration, |
18.0 |
23.0 |
16.5 |
17.0 |
|
Median PASI |
17.40 |
19.25 |
16.85 |
20.80 |
|
PASI >20 |
33.3 |
45.8 |
36.0 |
52.8 |
|
Median BSA |
20.0 |
26.0 |
21.0 |
22.5 |
|
IGA score |
21.6 |
35.4 |
30.0 |
41.5 |
|
Median DLQI |
13.0 |
12.0 |
13.0 |
15.0 |
|
Presence of PsA |
2.0 |
4.2 |
10.0 |
11.3 |
Efficacy1
The primary endpoint was met, with a significant reduction in Psoriasis Area and Severity Index (PASI) score with orismilast 20 mg (−53%; p < 0.001), 30 mg (−61%; p < 0.001), and 40 mg (−64%; p < 0.001) versus placebo (−17%) at Week 16.
PASI scores reduced up to 38% with orismilast 40 mg at Week 4 from baseline.
As per the multiple imputation (MI) and non-responder imputation (NRI) analysis, 20 mg and 40 mg doses showed significant improvement in PASI75 (≥75% reduction in PASI) and PASI90 (≥90% reduction in PASI; Figure 2).
Figure 2. PASI75 and PASI90 using A MI and B NRI analysis at Week 16 across orismilast doses (ITT population)*
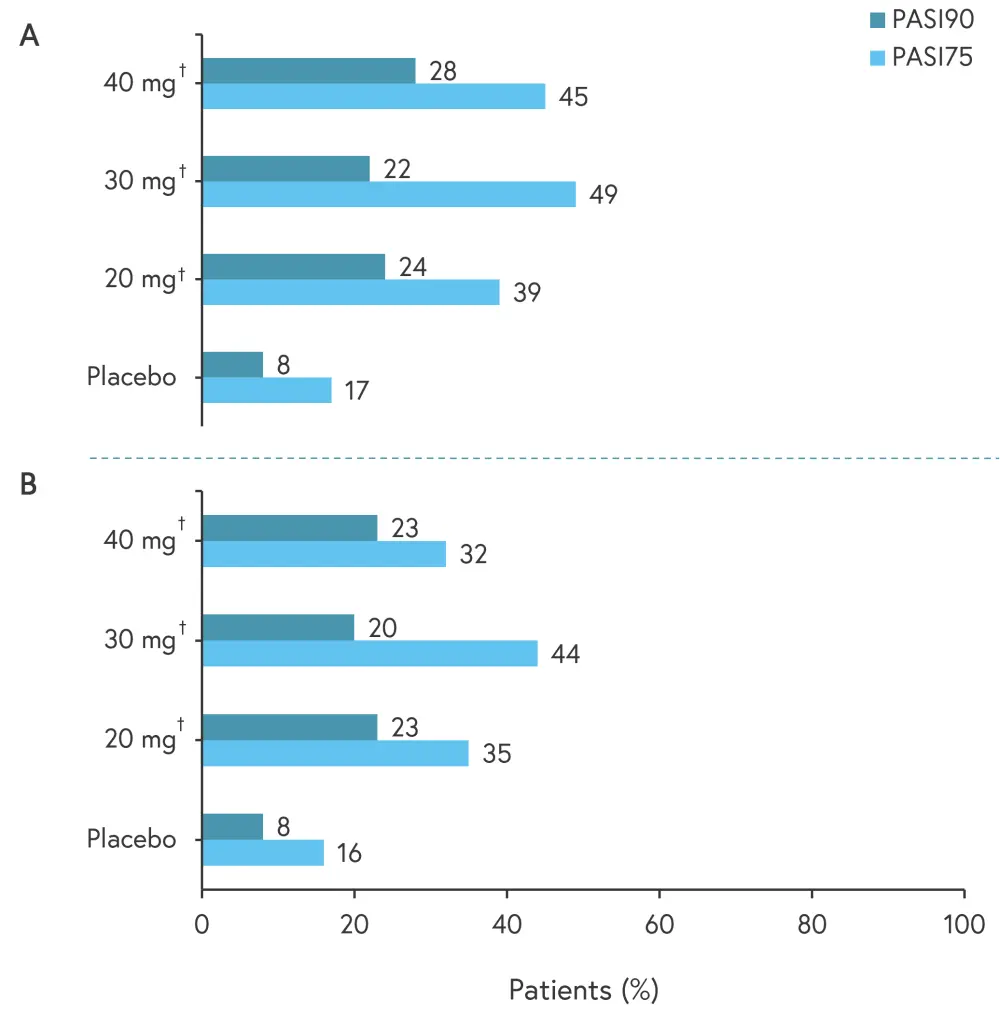
ITT, intent-to-treat; MI, multiple imputation; NRI, non-responder imputation; PASI, Psoriasis Area and Severity Index.
*Adapted from Warren, et al.1
†p < 0.05
In addition, 26%, 25%, and 21% of patients achieved Investigator’s Global Assessment scores of 0/1 with 20 mg, 30 mg, and 40 mg, respectively, versus 7% with placebo (p < 0.05).
Safety1
Dose dependent adverse events (AEs) were observed with orismilast (Table 2). Infections and depression rates were similar to placebo, with no malignancies or deaths reported.
Table 2. Safety findings*
|
TEAE, treatment-emergent adverse event. |
||||
|
Adverse event, % |
Placebo |
Orismilast |
||
|---|---|---|---|---|
|
20 mg |
30 mg |
40 mg |
||
|
Any TEAE |
45.1 |
77.1 |
84 |
94.3 |
|
TEAE by toxicity grade |
||||
|
Grade 1/mild |
33 |
52.1 |
72 |
75.5 |
|
Grade 2/moderate |
19.6 |
37.5 |
38 |
49.1 |
|
Grade 3 or more/severe |
3.9 |
16.7 |
14 |
15.1 |
|
TEAE leading to drug discontinuation |
3.9 |
20.8 |
20 |
39.6 |
|
Any serious TEAE |
0 |
2.1 |
2.0 |
0 |
|
Key TEAE |
||||
|
Infections |
17.6 |
16.7 |
22.0 |
15.1 |
|
Neoplasms benign, malignant, and unspecified |
0 |
0 |
0 |
0 |
|
Depression† |
2.0 |
0 |
2.0 |
0 |
|
Suicidal ideation† |
0 |
0 |
0 |
0 |
The most frequent AEs were diarrhea, nausea, and headache; these mainly occurred in the first month and were generally transient and mild in severity. The majority of these AEs did not lead to treatment discontinuation (Figure 3).
Figure 3. Most frequent TEAEs (reported in ≥5% of orismilast patients) by SOC and PT*
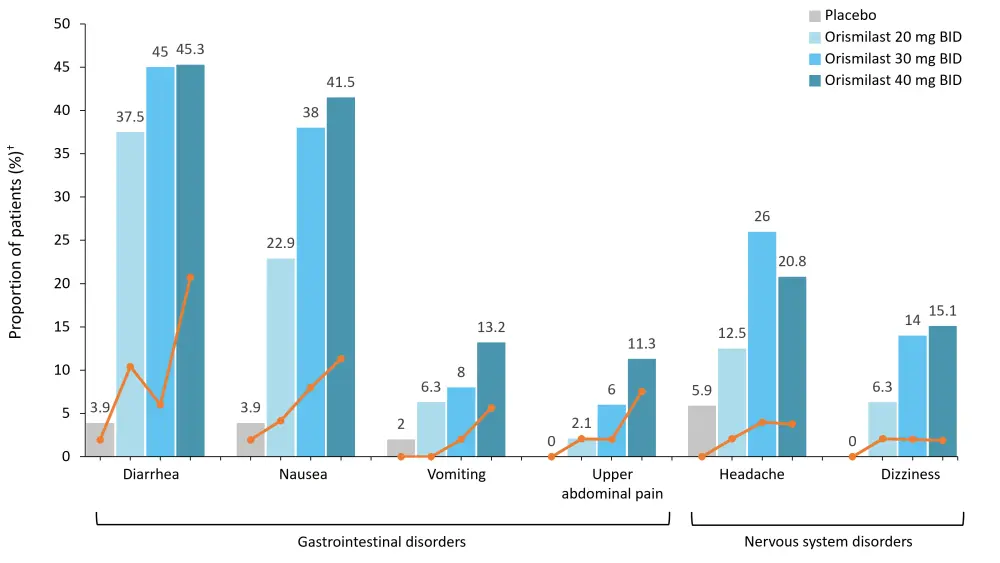
SOC, system organ class; PT, preferred term; TEAE, treatment-emergent adverse event.
*Adapted from Warren, et al.1
†Orange lines depict the proportion of patients discontinuing due to TEAE.
Conclusion1
In this phase IIb trial, orismilast modified-release tablets demonstrated significantly higher efficacy versus placebo in patients with moderate-to-severe psoriasis at Week 16. Safety and tolerability profiles were favorable and consistent with findings from other PDE-4 inhibitors. These results confirm the high potency of orismilast in inhibiting PDE4-B/D isoforms and demonstrate the potential of a new oral treatment regimen for psoriasis.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

