All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Izokibep in patients with active psoriatic arthritis: Latest results from a phase II study
Psoriatic arthritis (PsA) is a chronic, systemic inflammatory, autoimmune disease with a variety of symptoms, most prominently arthritis, dactylitis, and enthesitis, as well as plaque psoriasis and axial skeleton involvement. Current treatments focus on controlling inflammation and include disease-modifying antirheumatic drugs (DMARDs), nonsteroidal anti-inflammatory drugs (NSAIDs), and biologic agents that target different pathways of the immune system.1 However, there is still a risk of inadequate disease management and the development of new drugs remains urgent.
Interleukin-17A (IL-17A)-mediated immune response plays a pivotal role in PsA. Izokibep (ABY-035) is a novel small molecule IL-17A inhibitor with potent binding affinity for two subunits of IL-17A and serum albumin, allowing for a half-life extension and increased drug concentration in the sites of inflammation, including joints, skin, and nails.2 Izokibep was investigated in a phase II clinical trial (NCT04713072) to evaluate its efficacy and safety compared with placebo in patients with active PsA. Frank Behrens presented the first results from this trial at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress2 and we are pleased to provide a summary here.
Study design and patient characteristics
The study was a multicenter, randomized, double-blind, placebo-controlled, and dose-finding clinical trial to investigate the efficacy, safety, pharmacokinetics, and immunogenicity of izokibep versus placebo in patients with active PsA.
A total of 135 patients with a mean age of 48.5 years were enrolled at 22 European sites between June 2020 and July 2021. The patients were randomized to 40 mg izokibep or 80 mg izokibep or placebo (all biweekly) in a 1:1:1 ratio, and ≥50% improvement based on the American College of Rheumatology (ACR) criteria (ACR50) at Week 16 was evaluated as the primary endpoint. In total, 80% of patients received a concomitant conventional synthetic DMARD during the trial. The mean duration of PsA and psoriasis was ~8 years and ~10 years, respectively. Demographics and disease characteristics were balanced among the groups.
Study design and patient characteristics are detailed in Figure 1 and Table 1, respectively.
Figure 1. Study design*
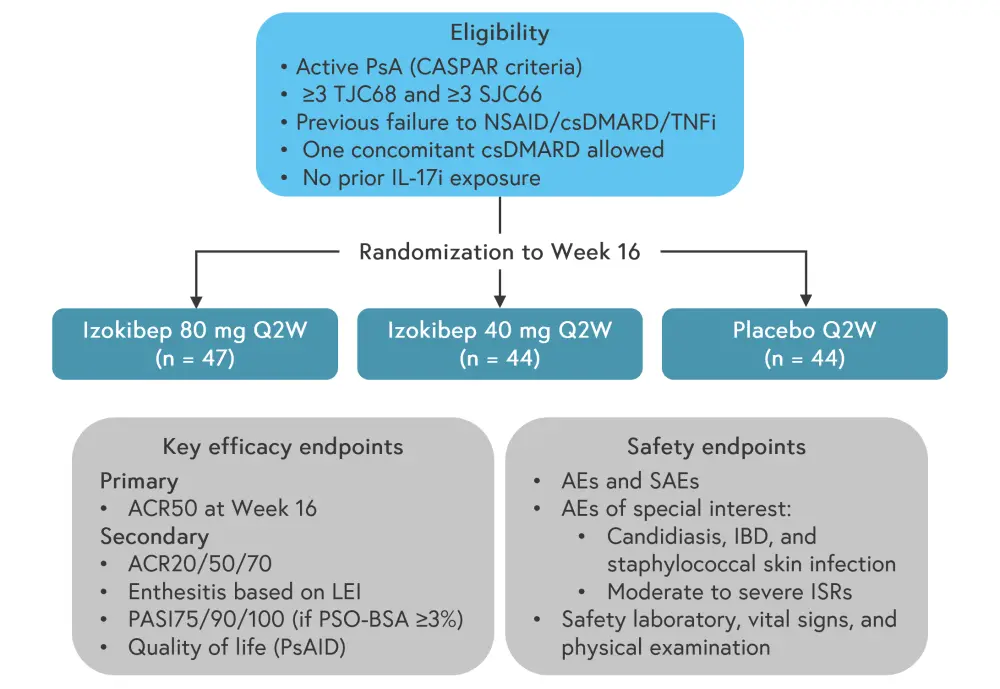
ACR, American College of Rheumatology; AE, adverse event; PSO-BSA, psoriasis body surface area; CASPAR, Classification Criteria for Psoriatic Arthritis; csDMARD, conventional synthetic disease modifying antirheumatic drug; IBD, inflammatory bowel disease; ISR, injection site reaction; LEI, Leeds Enthesitis Index; NSAID, non-steroidal anti-inflammatory drug; PASI, Psoriasis Area Severity Index; PsA, psoriatic arthritis; PsAID, Psoriatic Arthritis Impact of Disease; Q2W, once every two weeks; SJC66, Swollen Joints out of 66 joints; SAE, severe adverse event; TJC68, Tender Joints out of 68 joints; TNFi, tumor necrosis factor inhibitor.
*Adapted from Behrens.2
Table 1. Baseline characteristics*
|
BMI, body mass index; BSA, body surface area; csDMARD, conventional synthetic disease modifying antirheumatic drug; DAS28-CRP, Disease Activity Score 28 joint count calculated using C-reactive protein; HAQ-DI, Health Assessment Questionnaire-Disability Index; LEI, Leeds Enthesitis Index; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsAID, Psoriatic Arthritis Impact of Disease; SD, standard deviation. |
|||
|
Characteristic |
Izokibep 40 mg† |
Izokibep 80 mg† |
Placebo† |
|---|---|---|---|
|
Mean age, years (SD) |
48 (13) |
50 (11) |
48 (13) |
|
Female, % |
52 |
60 |
50 |
|
Mean BMI, kg/m2 (SD) |
30 (5.2) |
29 (4.5) |
28 (4.7) |
|
Mean duration of PsA, years (SD) |
7 (8) |
6 (6) |
8 (9) |
|
Mean duration of psoriasis, years (SD) |
17 (12) |
19 (14) |
19 (15) |
|
Concomitant csDMARD |
80 |
81 |
80 |
|
Previous TNFi |
11 |
17 |
9 |
|
Mean TJC68 (SD) |
17 (10) |
17 (10) |
16 (11) |
|
Mean SJC66 (SD) |
10 (7) |
10 (6) |
9 (6) |
|
Enthesitis LEI, % |
36 |
36 |
23 |
|
Mean DAS28-CRP (SD) |
4.5 (1.0) |
4.5 (1.1) |
4.5 (0.9) |
|
Psoriasis BSA >3%, % |
52 |
60 |
52 |
|
Mean PASI score (SD)‡ |
10 (7) |
8 (5) |
11 (7) |
|
Mean PsAID score (SD) |
5.8 (2.1) |
6.0 (1.6) |
5.7 (1.7) |
|
HAQ-DI (SD) |
1.2 (0.6) |
1.3 (0.5) |
1.2 (0.6) |
Results
Safety
Treatment-emergent adverse events were reported in 58% of patients and were mostly mild or moderate in severity. No serious adverse events or new onset/flare of inflammatory bowel disease were reported. Two patients discontinued the treatment due to injection site reactions. There were no apparent differences in safety between treatment groups and placebo, with the exception of injection site reactions in the izokibep 40 mg arm. Of note, there was only one mild vulvovaginal candida infection reported, which did not lead to treatment discontinuation. The results of the safety analysis are provided in Table 2.
Table 2. Safety outcomes*
|
AE, adverse event; TEAE, treatment emergent adverse event. |
|||
|
AE, % |
Izokibep 40 mg |
Izokibep 80 mg |
Placebo |
|---|---|---|---|
|
All TEAEs |
65.9 |
55.3 |
52.3 |
|
Mild |
54.5 |
51.1 |
40.9 |
|
Moderate |
25.0 |
6.4 |
27.3 |
|
TEAEs leading to trial withdrawal |
4.5† |
0 |
0 |
|
Preferred term (≥5%)‡ |
|
|
|
|
Injection site reaction |
29.5 |
25.5 |
0 |
|
Injection site erythema |
15.9 |
10.6 |
0 |
|
Headache |
0 |
8.5 |
9.1 |
|
Hypertension |
4.5 |
0 |
9.1 |
|
Diarrhea |
0 |
4.3 |
6.8 |
|
Hyperkalemia |
6.8 |
4.3 |
4.5 |
|
Upper respiratory tract infection |
4.5 |
6.4 |
2.3 |
|
Other AEs of interest |
|
|
|
|
COVID-19 infection |
2.3 |
2.1 |
4.5 |
|
Candidiasis |
2.3§ |
0 |
0 |
Efficacy
At 16 weeks, the ACR50 response rates were 48% and 52% in the 40 mg and 80 mg groups, respectively, compared with 13% in the placebo group (p = 0.0014 and p = 0.0006, respectively). The ACR20/50/70 response rates by treatment group are presented in Figure 2A. As shown in Figure 2B, both treatment groups demonstrated greatly improved response rates in terms of the ≥75% and ≥90% improvement in Psoriasis Area and Severity Index (PASI-75 and PASI-90, respectively) in patients with a psoriasis body surface area of >3% (izokibep 40 mg, n = 23; izokibep 80 mg, n = 28; and placebo, n = 23).
Figure 2. Response rates according to A ACR and B PASI scores at 16 weeks*
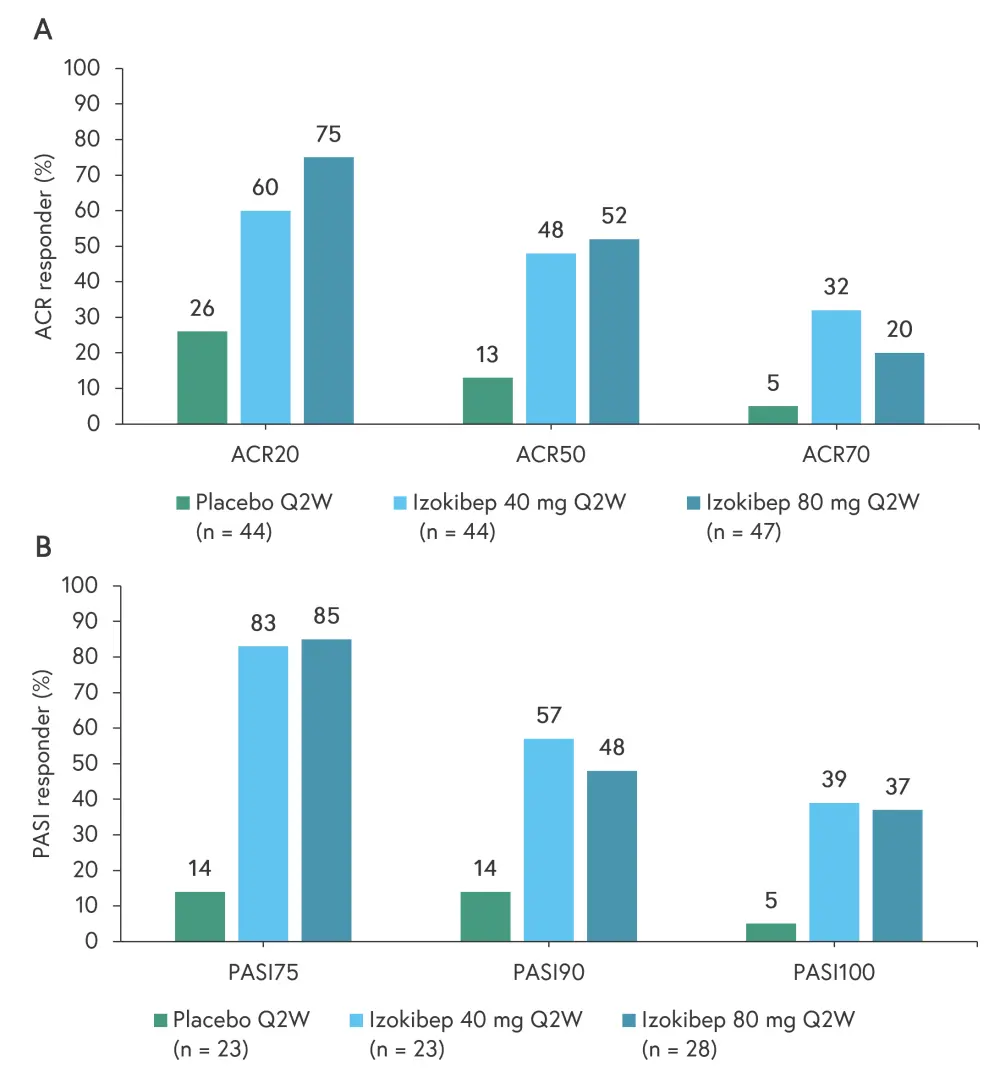
ACR, American College of Rheumatology; ACR20, ≥20% improvement based on ACR criteria; ACR50, ≥50% improvement based on ACR criteria; ACR70, ≥70% improvement based on ACR criteria; PASI, Psoriasis Area and Severity Index; PASI75, ≥75% improvement in PASI; PASI90, ≥90% improvement in PASI; PASI100, ≥100% improvement in PASI; Q2W, once every two weeks.
*Data from Behrens.2
In addition, resolution of enthesitis (Leeds Enthesitis Index = 0) at Week 16 was 63% for the 40 mg group, 88% for the 80 mg group, and 10% for the placebo group in those with enthesitis at baseline. There were also clinically important improvements in quality of life according to the Psoriatic Arthritis Impact of Disease score at 16 weeks, with the proportion of patients achieving a minimal clinical important improvement at 41% in the 80 mg group versus 12% in the placebo group.
Conclusion
Overall, the results of this study suggest that 16 weeks of izokibep treatment was associated with high response rates (ACR50, PASI75, and PASI90) in patients with PsA, showing a dose-dependent efficacy in the musculoskeletal and cutaneous domains. Izokibep was generally well tolerated and its safety profile was broadly consistent with placebo in this study and with approved IL-17A inhibitors in general. No dose-related adverse events or dose-limiting toxicity were reported, suggesting izokibep could be tested at higher doses. Further larger clinical studies are under development.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

