All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Deucravacitinib for the treatment of psoriatic arthritis
Deucravacitinib is a novel, oral, selective TYK2 inhibitor recently approved by the U.S. Food and Drug Administration (FDA) for the treatment of adults with moderate to severe plaque psoriasis. In addition to showing efficacy in treating psoriasis, deucravacitinib has shown efficacy in a phase II trial in patients with systemic lupus erythematosus.1
The Psoriasis and Psoriatic Arthritis Hub has previously reported on the long-term efficacy of deucravacitinib and COVID-19-related adverse events in patients with plaque psoriasis; please see this previous article for information on the mechanism of action,.
At the American College of Rheumatology (ACR) annual meeting (ACR Convergence 2022), Mease reported data from a phase II trial (NCT03881059) assessing the dose-response relationship of deucravacitinib in patients with psoriatic arthritis, with a focus on Part B of the trial, which involved treatment up to Week 52. We are pleased to provide a summary of this presentation here.
Study design
The study design is shown in Figure 1. Patients were initially randomized to either placebo or deucravacitinib 6 mg or 12 mg. If patients met minimal disease activity (MDA) at the end of 16 weeks of treatment with deucravacitinib, they were able to remain on deucravacitinib in Part B of the trial. If MDA was not met, patients were transferred to ustekinumab in Part B. Both Part A and B were blinded.
Figure 1. Study design*
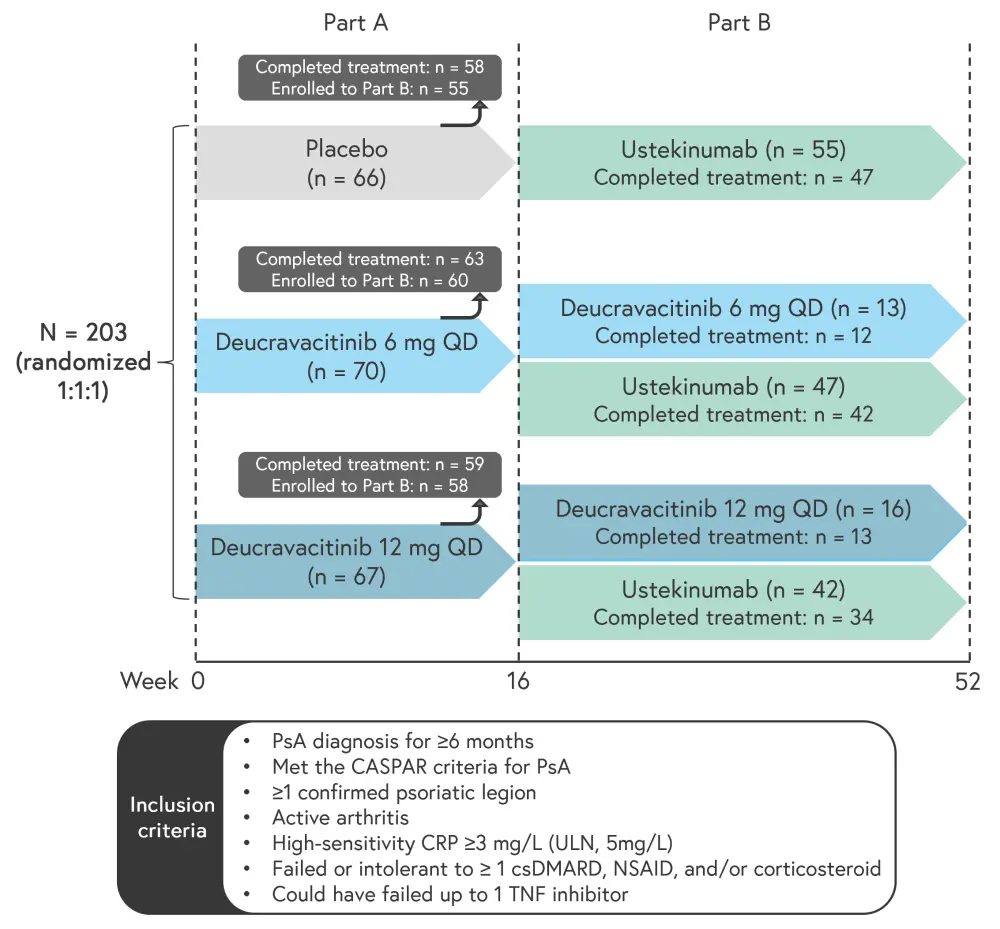
CRP, C-reactive protein; csDMARD, conventional synthetic disease modifying antirheumatic drug; CASPAR, Classification of Psoriatic Arthritis; NSAID, non-steroidal anti-inflammatory drug; PsA, psoriatic arthritis; QD, once daily; TNF, tumor necrosis factor.
*Adapted from Mease.1
The primary endpoint of Part A of the study was ACR20 at Week 16, with secondary endpoints of HAQ-DI (Health Assessment Questionnaire Disability Index), PASI-75 (≥75% improvement in Psoriasis Area Severity Index from baseline), and SF-36 (36-Item Short Form Health Survey) scores at Week 16. Exploratory endpoints at Week 52 included patient-reported outcomes, PASDAS (Psoriatic Arthritis Disease Activity Score), and DAPSA (Disease Activity in Psoriatic Arthritis).
Results
Safety
The occurrence of adverse events in Part A (0–16 weeks) is shown in Table 1, with adverse events in Part B (16–52 weeks) shown in Table 2. During Part A, 65.7% of patients in the deucravacitinib arms reported adverse events, compared with 42.4% in the placebo arm (Table 1). In Part B, 84.6% of patients treated with 12 mg deucravacitinib experienced adverse events, compared with 61.9% in the deucravacitinib/ustekinumab arm. A total of 6.9% and 4.8% discontinued treatment in deucravacitinib and deucravacitinib/ustekinumab arms, respectively (Table 2).
Table 1. Adverse events in Part A*
|
*Adapted from Mease.1 |
|||
|
Adverse event, % |
Placebo |
Deucravacitinib 6 mg |
Deucravacitinib 12 mg |
|---|---|---|---|
|
All adverse events |
42.4 |
65.7 |
65.7 |
|
Serious adverse events |
1.5 |
0 |
0 |
|
Discontinued due to adverse event |
1.5 |
4.3 |
6.0 |
|
Death |
0 |
0 |
0 |
|
Most common adverse events |
|
|
|
|
Nasopharyngitis |
7.6 |
5.7 |
17.9 |
|
Sinusitis |
0 |
0 |
7.5 |
|
Headache |
4.5 |
7.1 |
1.5 |
Table 2. Adverse events in Part B*
|
AST, aspartate aminotransferase; deuc, deucravacitinib. †Female aged 70 years; sudden death on Day 325. |
|||||
|
Adverse event, % |
Placebo/ ustekinumab |
Deuc 6mg |
Deuc 12mg |
Deuc 6 mg/ ustekinumab |
Deuc 12 mg/ ustekinumab |
|---|---|---|---|---|---|
|
All adverse events |
54.5 |
84.6 |
50.0 |
55.3 |
61.9 |
|
Serious adverse events |
0 |
7.7 |
0 |
6.4 |
9.5 |
|
Discontinued due to adverse event |
0 |
0 |
6.3 |
0 |
4.8 |
|
Death |
0 |
0 |
0 |
2.1† |
2.4‡ |
|
Most common adverse events |
|
|
|
|
|
|
Pyrexia |
0 |
7.7 |
18.8 |
0 |
2.4 |
|
AST |
0 |
15.4 |
6.3 |
0 |
4.8 |
|
COVID-19 |
0 |
0 |
6.3 |
2.1 |
11.9 |
Efficacy
The mean patient reported change in PASI score from baseline for the patients who received deucravacitinib through to Week 52 was −9.9 in patients who received 6 mg and −8.3 in patients who received 12 mg. Mean PASDAS and DAPSA scores from baseline to Week 52 in all treatment groups are shown in Figure 2 and Figure 3.
Figure 2. Mean PASDAS scores from baseline to Week 52*
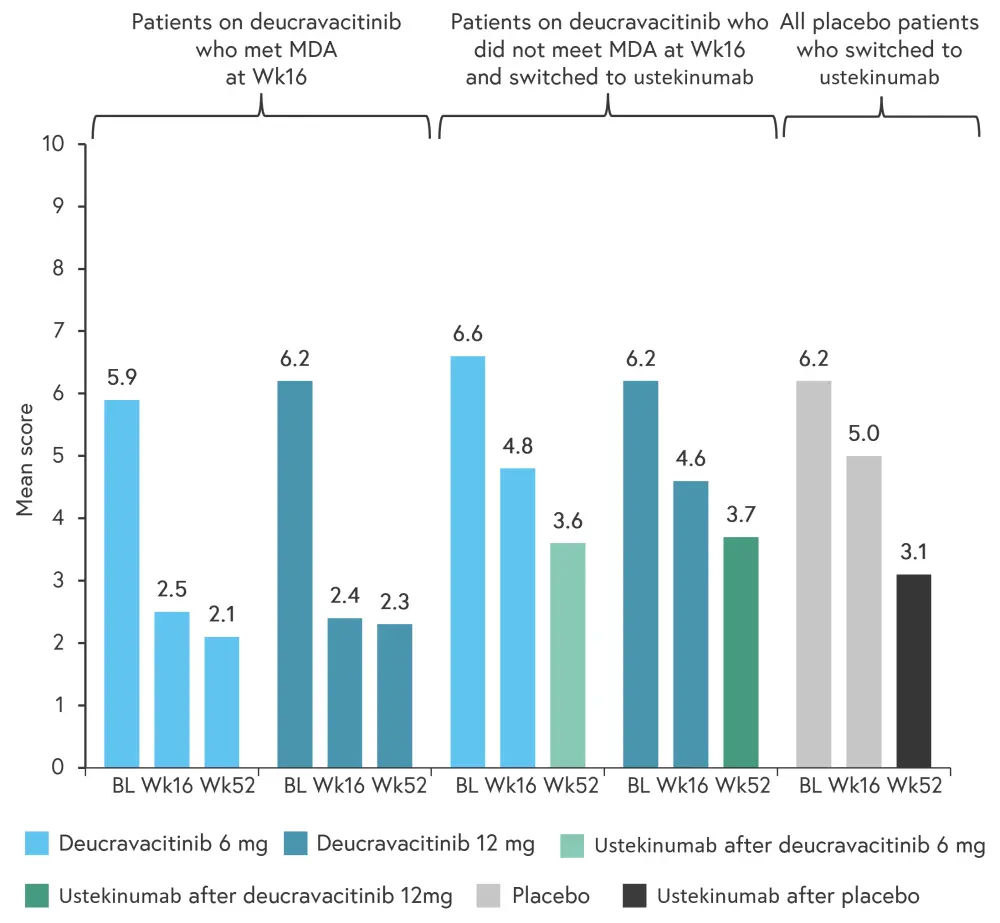
BL, baseline; MDA, minimal disease activity; PASDAS, Psoriatic Arthritis Disease Activity Score; Wk, week.
*Adapted from Mease1
Patients treated with deucravacitinib through to Week 52 saw an ~3.5-point mean change in PASDAS score by Week 16 that was maintained to Week 52. Patients treated with deucravacitinib who did not meet MDA by Week 16 and placebo patients who switched to ustekinumab saw an ~3-point mean change in PASDAS by Week 52.
Figure 3. Mean DAPSA scores from baseline to Week 52*
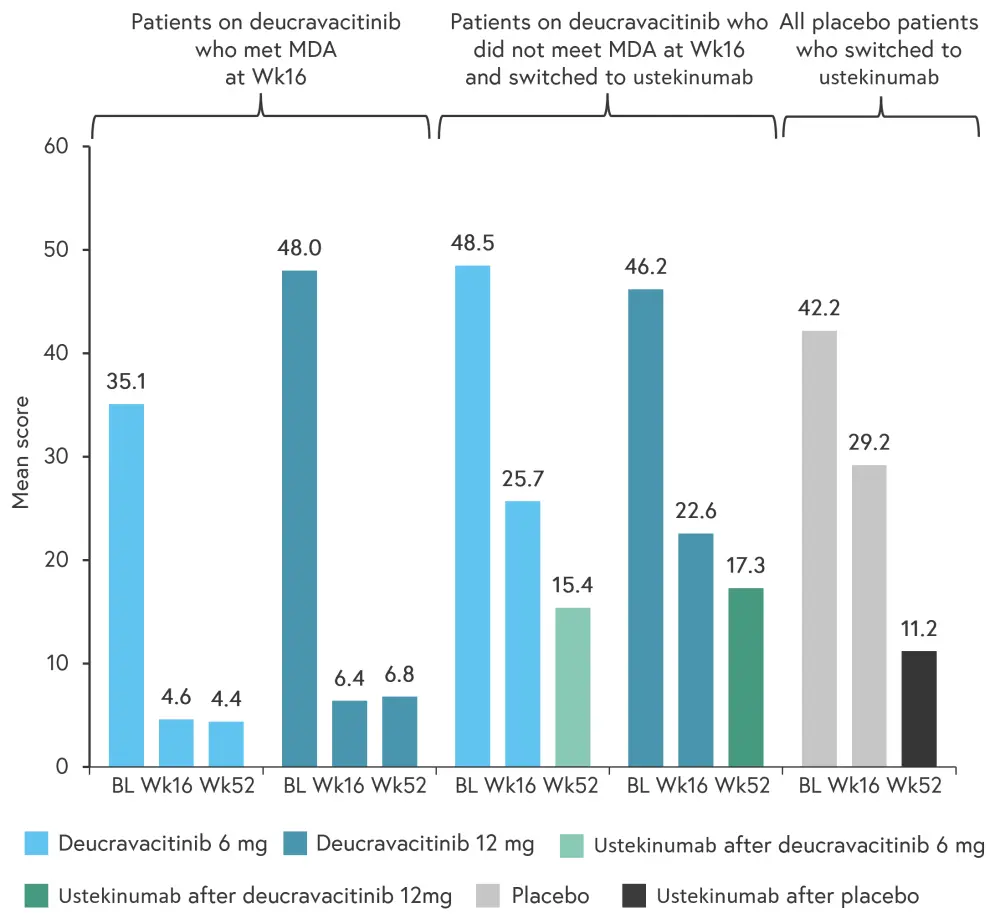
BL, baseline; DAPSA, Disease Activity in Psoriatic Arthritis; MDA, minimal disease activity; Wk, week.
*Adapted from Mease1
DAPSA scores >28 are considered high, with scores 15−28, 5−14, and 0−4 considered moderate, low, and remission, respectively. Patients who were treated with deucravacitinib for 52 weeks went from a mean DAPSA score of high to a mean score of low that almost reached remission. Patients treated with ustekinumab also had a reduction in the mean score, although this reduction was not as significant.
Conclusion
Deucravacitinib showed an acceptable safety profile in this trial, with no new safety signals observed in Part B of the study. The improvements shown in PASDAS and DAPSA at Week 16 and Week 52 in patients treated with deucravacitinib suggest that it is an effective and safe option for the treatment of active psoriatic arthritis. There is currently an ongoing phase III study of deucravacitinib in patients with psoriatic arthritis that will provide further efficacy and safety data.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

