All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
5-year safety assessment of apremilast in patients with psoriasis and psoriatic arthritis
Introduction
Apremilast, a small molecule inhibitor of phosphodiesterase-4 (involved in inflammatory signaling pathways), has been used to treat patients with psoriasis (Pso) or psoriatic arthritis (PsA). Following the European Medicine’s Agency (EMA) approval of apremilast for the treatment of adults with moderate-to-severe plaque psoriasis in 2015, a post-authorization safety study was requested.
Here, we summarize results from the 5-year longitudinal study by Persson et al.1 examining safety signals in UK patients treated with apremilast compared with oral only, injectable only, or oral plus injectable treatments.
Study design
This was a prospective cohort study conducted using data from the UK Clinical Practice Research Datalink, the study design is shown in Figure 1.
Figure 1. Study design*
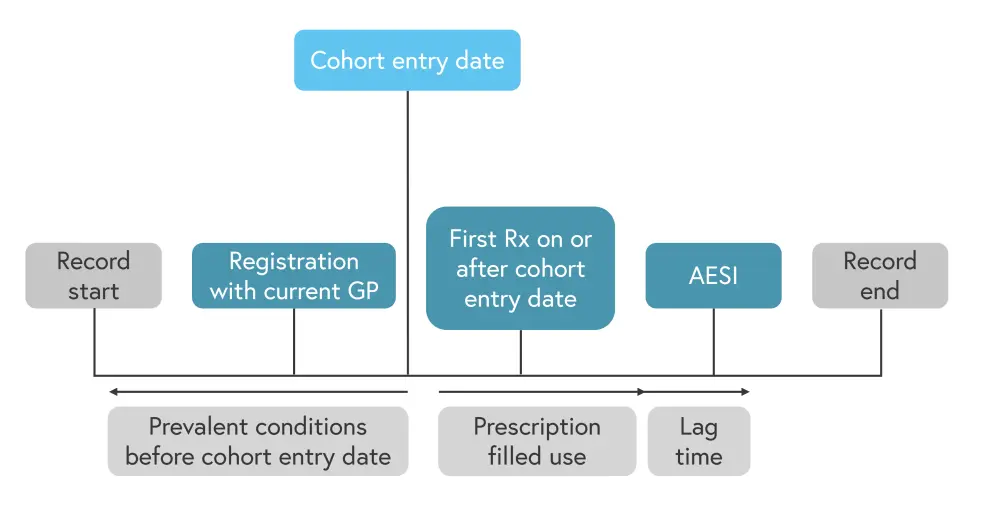
AESI, adverse events of special interest; GP, general practitioner; Rx, prescription.
*Adapted from Persson, et al.1
The four exposure groups included:
- apremilast
- oral only
- injectable only
- oral plus injectable
Each patient in the apremilast group was matched to ≤10 patients in the non-apremilast group for age (± 3 years), sex, year of record start (± 3 years or a longer history for the matches than the apremilast-exposed patient), and calendar time.
Results
As shown in Table 1, 341 apremilast treated patients were matched with non-apremilast patients with Pso or PsA. A decreased percentage of patients with PsA were included in the apremilast group compared with the oral plus injectable exposure group and comorbidity distribution was similar between the apremilast exposed and non-exposed groups.
Table 1. Baseline patient characteristics*
|
BMI, body mass index; GP, general practitioner; MACE, major adverse cardiac events; PsA, psoriatic arthritis; Pso, psoriasis; SD, standard deviation. ‡Patients may have had more than one malignancy at cohort entry. §Record contains at least one prescription for an antidepressant recorded within 60 days of a depression diagnosis, with both treatment and diagnosis codes recorded before the cohort entry date. |
||||
|
Characteristic, % (unless |
Apremilast |
Non-apremilast |
||
|---|---|---|---|---|
|
Oral only |
Injectable |
Oral + |
||
|
Mean age (SD), years |
53 (±14) |
53 (±14) |
58 (±13) |
55 (±15) |
|
Female |
58 |
58 |
55 |
60 |
|
Male |
42 |
42 |
45 |
40 |
|
Diagnosis |
|
|
|
|
|
PsA with or |
44 |
42 |
31 |
65 |
|
Pso only |
50 |
58 |
69 |
35 |
|
Neither diagnosis |
6 |
0 |
0 |
0 |
|
Smoking status |
|
|
|
|
|
Current |
22 |
24 |
18 |
18 |
|
Former |
36 |
39 |
45 |
46 |
|
Non-smoker |
39 |
38 |
36 |
36 |
|
Unknow smoking |
3 |
1 |
1 |
<1 |
|
BMI before cohort entry, |
|
|
|
|
|
<18.5 |
1 |
2 |
1 |
1 |
|
18.5 to <25.0 |
16 |
21 |
20 |
18 |
|
25.0 to <30.0 |
25 |
31 |
32 |
31 |
|
≥30.0 |
49 |
39 |
42 |
45 |
|
Unknown |
8 |
7 |
6 |
5 |
|
Mean time between |
16 (±9) |
17 (±8) |
19 (±8) |
18 (±8) |
|
Prevalence of |
|
|
|
|
|
MACE |
4 |
4 |
4 |
4 |
|
Tachyarrhythmias |
5 |
5 |
5 |
5 |
|
Vasculitis |
1 |
1 |
0 |
1 |
|
Malignancy‡ |
9 |
8 |
11 |
10 |
|
Solid |
8 |
5 |
7 |
7 |
|
Hematologic |
<1 |
1 |
1 |
<1 |
|
Non-melanoma |
2 |
3 |
4 |
3 |
|
Treated |
20 |
23 |
23 |
23 |
|
Treated anxiety‖ |
14 |
19 |
18 |
21 |
|
Suicidal behaviors |
9 |
9 |
7 |
9 |
|
Acute comorbidities in the |
|
|
|
|
|
Opportunistic |
3 |
3 |
3 |
4 |
|
Hypersensitivity |
4 |
2 |
1 |
3 |
Patients treated with both injectable and oral treatments had more than double the number of treatments compared with the other exposure groups (Table 2). Apremilast was used in combination with another agent (often methotrexate or prednisolone) in 26% of patients.
Table 2. Treatment characteristics of patients in the apremilast and non-apremilast cohorts*
|
SD, standard deviation. |
||||
|
Characteristic, % |
Apremilast |
Non-apremilast |
||
|---|---|---|---|---|
|
Oral only |
Injectable only |
Oral + injectable |
||
|
Length of medical record after cohort entry† |
||||
|
Mean (SD) |
21 (±15) |
22 (±15) |
30 (±16) |
31 (±16) |
|
<6 months |
15 |
14 |
6 |
5 |
|
6 months to <2 years |
46 |
46 |
33 |
29 |
|
2 to <4 years |
30 |
31 |
43 |
44 |
|
>4 years |
9 |
9 |
18 |
22 |
|
Number of study drug prescriptions on or after cohort entry‡ |
||||
|
Mean (SD) |
8 (±10) |
7 (±10) |
4 (±8) |
19 (±21) |
|
1 |
29 |
32 |
64 |
0 |
|
2 |
9 |
13 |
11 |
9 |
|
3–5 |
17 |
19 |
10 |
17 |
|
6–9 |
17 |
12 |
4 |
13 |
|
≥10 |
28 |
23 |
11 |
62 |
|
Study drugs used by ≥5% of patients in any cohort during the follow-up |
||||
|
Apremilast |
100 |
0 |
0 |
0 |
|
Leflunomide |
1 |
5 |
0 |
10 |
|
Methotrexate |
12 |
31 |
21 |
67 |
|
Methylprednisolone |
1 |
<1 |
45 |
29 |
|
Prednisolone |
11 |
57 |
0 |
46 |
|
Sulfasalazine |
3 |
14 |
0 |
25 |
|
Triamcinolone |
1 |
0 |
31 |
15 |
The apremilast group showed low levels of adverse events of special interest (AESI), with 18 infections and 11 hypersensitivity reactions recorded in the cohort of 341 patients (Table 3). No cases of vasculitis, blood cancer, non-melanoma skin malignancy or treated anxiety, depression, or suicidal behaviors were recorded for the apremilast group during the ≤59-month follow-up.
Incidence of major adverse cardiac events, tachyarrhythmias, solid malignancies was similar across all treated cohorts, as seen in Table 3. Patients treated with oral medicine only had the highest recorded number of events for each AESI. Patients treated with injectables had <5 events recorded for all AESI except all-cause death for which 6 events were noted.
Table 3. Incidence rate and incidence rate ratios of AESIs*
|
AESI, adverse events of special interest; CI, confidence interval; IR, incidence rate; IRR, incidence rate ratio; MACE, major adverse cardiac events; PY, person-years. |
||||
|
Treatment cohort, n (unless otherwise stated) |
Events |
PY |
IR per 1000 PY |
Crude IRR |
|---|---|---|---|---|
|
Opportunistic and serious infections‡ |
||||
|
Apremilast |
18 |
282 |
64 (40–102) |
ref. |
|
Oral |
119 |
2,370 |
50 (42–60) |
0.8 (0.5–1.3) |
|
injectable |
8 |
394 |
20 (10–41) |
0.3 (0.1–0.7) |
|
Oral + injectable |
105 |
1,853 |
57 (47–69) |
0.9 (0.5–1.5) |
|
Hypersensitivity reactions‡ |
||||
|
Apremilast |
11 |
281 |
39 (22–71) |
ref. |
|
Oral |
169 |
2324 |
73 (63–85) |
1.9 (1.0–3.4) |
|
Injectable |
19 |
388 |
49 (31–77) |
1.2 (0.6–2.6) |
|
Oral + injectable |
117 |
1849 |
63 (53–76) |
1.6 (0.9–3.0) |
|
MACE |
||||
|
Apremilast |
<5§ |
281 |
7 (2–29) |
— |
|
Oral |
10 |
2,343 |
4 (2–8) |
— |
|
Injectable |
<5 |
393 |
10 (4–27) |
— |
|
Oral + injectable |
6 |
1,911 |
3 (1–7) |
— |
|
Tachyarrhythmias |
||||
|
Apremilast |
<5 |
273 |
11 (4–34) |
— |
|
Oral |
10 |
2,350 |
4 (2–8) |
— |
|
Injectable |
<5 |
378 |
5 (1–21) |
— |
|
Oral + injectable |
7 |
1,900 |
4 (2–8) |
— |
|
Solid malignancies |
||||
|
Apremilast |
<5 |
267 |
8 (2–30) |
— |
|
Oral |
13 |
2,293 |
6 (3–10) |
— |
|
Injectable |
<5 |
377 |
11 (4–28) |
— |
|
Oral + injectable |
10 |
1,859 |
5 (3–10) |
— |
|
All-cause mortality |
||||
|
Apremilast |
<5 |
292 |
7 (2–27) |
— |
|
Oral |
61 |
2,471 |
25 (19–32) |
— |
|
Injectable |
6 |
404 |
15 (7–33) |
— |
|
Oral + injectable |
42 |
2,005 |
21 (16–28) |
— |
Conclusion
The number of AESI in patients with Pso and/or PsA for the apremilast group was low compared with the non-apremilast treatment groups, with no new safety signals recorded. While the rate of opportunistic infections in the apremilast group was slightly higher than the injectable only group, rates were similar in the oral and oral + injectable groups. This 5-year prospective UK study demonstrated that the safety profile of apremilast in a real-world setting was comparable to what has been reported in clinical trials for patients with Pso and/or PsA.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

