All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, founding supporter. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Validity of the PBI-TOP tool for assessing patient goals and benefits of topical treatment in psoriasis
Topical treatment is often used as a first-line therapy for mild-to-moderate psoriasis and is available as cream, ointment, gel, foam, and lotion.1 Topical treatment works by reducing inflammation and reducing skin cell growth, but it can be less effective in patients with severe symptoms.1 Additionally, topical treatment can require increased effort and time to apply, which may result in low adherence.1
Here, we summarize a pilot validation study by Blome et al.1 published in British Journal of Dermatology assessing the feasibility of the Patient Benefit Index for Topical Treatment (PBI-TOP) tool in measuring the benefits of topical treatment in adult patients with psoriasis.1
Study design1
- The following themes were identified through patient focus groups, patient interviews, open surveys, and literature searches:
-
- Benefit of treatment
- Effectiveness on symptoms
- Effectiveness on quality of life
- Characteristics of the preparation
- Benefit of treatment
- Based on these themes, a pilot version of PBI-TOP was created, consisting of two sections:
- Patient needs questionnaire (PNQ), where patients rate the importance of treatment goals in current/upcoming treatment
- Patient benefit questionnaire (PBQ), where patient rate whether treatment goals have been achieved
- Using both sections, a weighted score is calculated that shows treatment benefit for the patient.
Key findings1
- A total of 154 patients completed the PNQ.
- Of these, 121 also completed the PBQ and were included in treatment goal and benefit analysis in addition to assessing pilot validity.
- Achievement of items, as assessed by PBQ, with the highest mean importance in the therapy goals and characteristics preferences in PNQ are shown in Figure 1. The percentage of patients who agreed they were quite/very important, were:
- Less dry, cracked or scaly skin (86.8%)
- Reduced redness or inflammation of the skin (86.0%)
- No visible skin changes (78.5%)
- No more itching (80.2%)
- In the preparation characteristics preferences section of PBI-TOP, items with the highest mean importance in the PNQ, and percentage of patients who agreed they were quite/very important, were:
- The preparation should be well tolerated (83.5%)
- The preparation should not stain objects (71.9%)
- The preparation should be easy to use (71.9%)
- The preparation should be easy to dose (68.6%)
- Items with the highest importance in the PNQ were not always highly achieved according to the PBQ (Figure 1). For example, ~60% or less of patients agreed that their therapy goals were quite or very much achieved after use of topical therapy, despite ~80% stating that this was quite/very important.
Figure 1. Achievement of the highest importance therapy goals and preparation characteristics (results of the PBQ)*
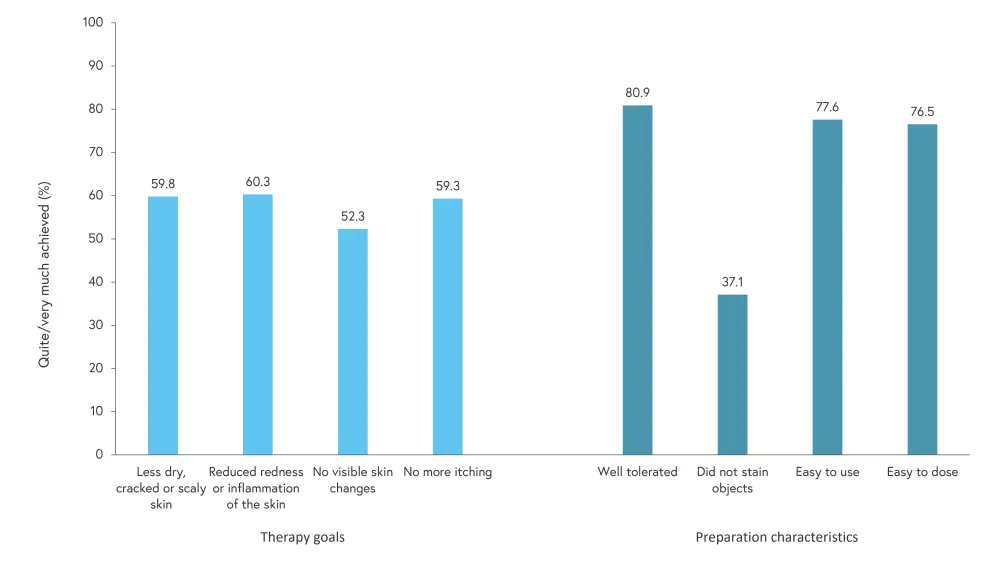
PBQ, patient benefit questionnaire.
*Data from Blome, et al.1
- Table 1 shows the internal consistency of PBI-TOP scale and its correlation with convergent criteria.
Table 1. PBI-TOP scale internal consistency and correlation with convergent criteria*
|
BSA, body surface area; DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index; PNQ, patient needs questionnaire; PBI-TOP, Patient Benefit Index, version for topical treatment; PBQ, patient benefit questionnaire; r, correlation coefficient. |
||||
|
PBI-TOP scale |
Internal consistency (Patients with PNQ data, n = 154) |
Correlation with convergent criteria (patients with PNQ and PBQ data, n = 121) |
||
|---|---|---|---|---|
|
|
Cronbach’s α |
DLQI r (p value)† |
PASI r (p value)† |
BSA r (p value)† |
|
Overall effectiveness score |
0.94 |
–0.41 (<0.001) |
–0.32 (0.001) |
–0.22 (0.046) |
|
Effectiveness subscale |
||||
|
Symptoms |
0.90 |
–0.41 (<0.001) |
–0.27 (0.007) |
–0.16 (0.155) |
|
Quality of life |
0.94 |
–0.32 (0.002) |
–0.33 (0.002) |
–0.23 (0.055) |
|
Characteristics of the preparation score |
0.90 |
–0.34 (0.001) |
–0.19 (0.053) |
–0.19 (0.089) |
|
Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

