All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
Sex differences in adverse drug reactions in patients with immune-mediated inflammatory diseases
Do you know... A recent Dutch study investigated the differences in the frequency and distribution of adverse drug reactions (ADRs) between male and female patients with rheumatoid arthritis, psoriatic arthritis, or axial spondyloarthritis following treatment with etanercept or adalimumab. Over 850 ADRs were reported, what percentage of those were reported by female patients?
Introduction
Adverse drug events, including adverse drug reactions (ADRs), account for nearly 5% of unplanned hospital admissions, increasing the burden on health services.1 Many patient-specific factors influence the occurrence of ADRs including genetics, pharmacokinetics, hormonal status, and gender.2
Women are twice as likely to experience ADRs compared with men; however, the role of sex differences in ADRs is not well understood. Recent interest in elucidating this association between sex and ADRs is due to disparities, not only in disease and ADR incidence between the two sexes, but more importantly in the effectiveness of drug therapies.1
During the 7th Congress of the Skin Inflammation & Psoriasis International Network (SPIN), van Lint3 presented the findings of a study examining sex differences in relation to the nature, frequency, and burden of patient-reported ADRs attributed to adalimumab or etanercept. The findings were also recently published in Annals of the Rheumatic Diseases.4
Study design3,4
This was a prospective cohort study in patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA), or axial spondyloarthritis (SpA) treated with etanercept or adalimumab. Patient-reported ADRs via bimonthly questionnaires and data from eligible patients were collected from the Dutch Biologic Monitor (DBM). Sex-specific ADRs, such as menstruation disorders, were excluded.
The primary outcomes included the nature, distribution, and frequency of ADRs, coded using the Medical Dictionary for Regulatory Activities (MedDRA). The secondary outcome was the burden of ADRs, measured using a 5-point Likert scale.
Results3,4
Baseline characteristics
A total of 748 patients with RA, PsA, or axial SpA were included; 48% of patients reported at least one ADR and 59% were female. The number of female patients reporting at least one ADR was statistically higher compared with male patients (55% vs 38%; p < 0.001). Table 1 summarizes the baseline characteristics.
Table 1. Baseline characteristics*
|
MTX, methotrexate; PsA, psoriatic arthritis; RA, rheumatoid arthritis; SD, standard deviation; SpA, axial spondyloarthritis. |
||
|
Characteristic, % (unless stated otherwise) |
Total (N = 748) |
|
|---|---|---|
|
Male (n = 304) |
Female (n = 444) |
|
|
Mean age (± SD)†, years |
58.2 (±11.9) |
56.6 (±12.9) |
|
Disease indication‡ |
||
|
Axial SpA |
23.4 |
8.8 |
|
Axial SpA and PsA |
1.3 |
1.8 |
|
Axial SpA and RA |
2.6 |
0.7 |
|
PsA |
29.3 |
18.9 |
|
RA |
43.4 |
69.8 |
|
Biologics |
||
|
Adalimumab |
45.4 |
44.8 |
|
Etanercept |
52.3 |
52.3 |
|
Co-medication§ |
|
|
|
Aminosalicylates |
4.6 |
7.4 |
|
Corticosteroids |
8.9 |
11.0 |
|
MTX |
35.2 |
43.7 |
|
Thiopurines |
1.0 |
2.2 |
Distribution and frequency
A total of 882 ADRs were reported, including 264 different types of ADR, with most reported by female patients (74%). The distribution of ADRs was statistically significant for female patients compared with male patients (p = 0.025). Figure 1 shows the nature and distribution of the ADRs reported by male and female patients.
Figure 1. Distribution and nature of ADRs*
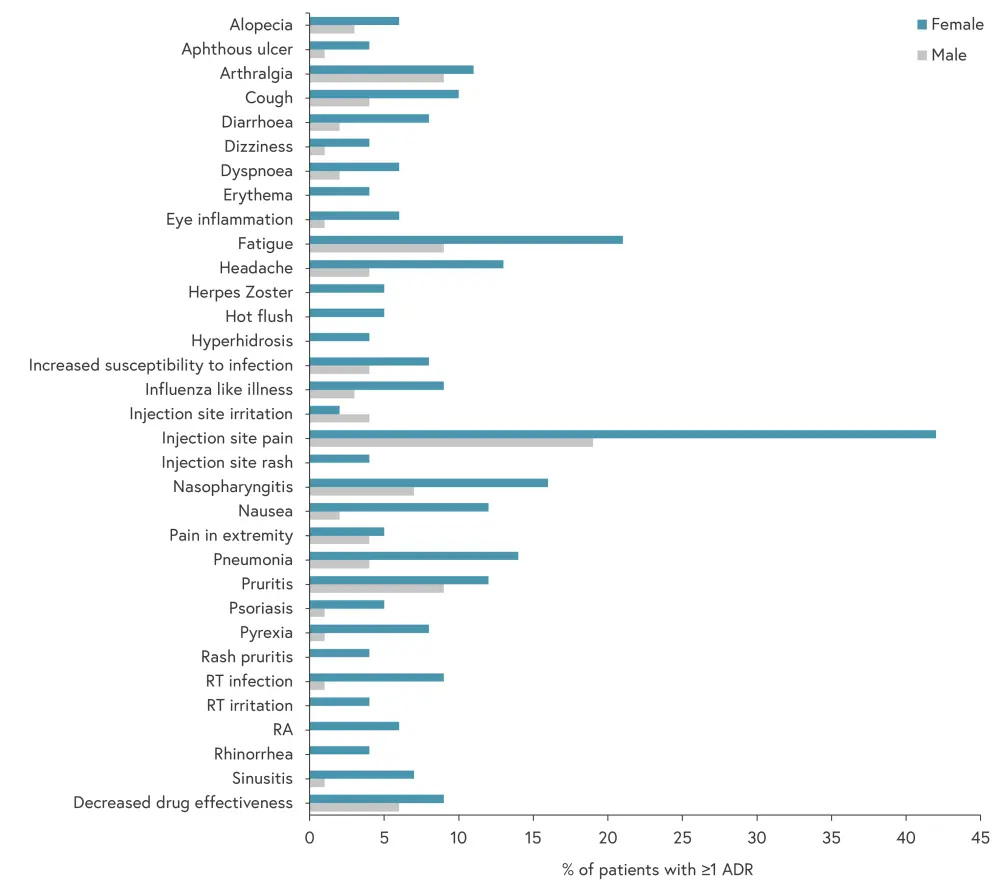
ADR, adverse drug reaction; RA, rheumatoid arthritis; RT, respiratory tract.
*Adapted from van Lint.3
The distribution of ADRs differed between male and female patients for pruritis, inflammation and hematoma at the injection site, erythema, hematoma, and cystitis (Figure 2). However, following correction for multiple testing, these differences were no longer statistically significant.
Figure 2. Differences in the distribution of ADRs*
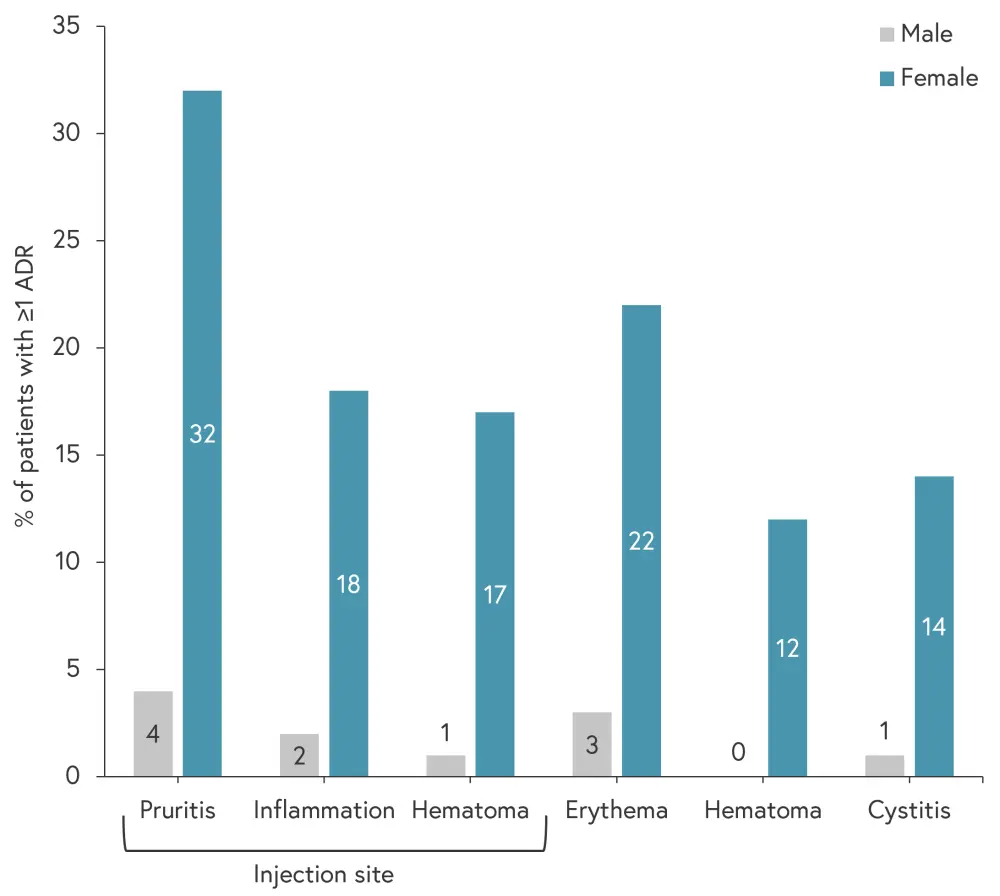
ADRs, adverse drug reaction.
*Adapted from Gosselt, et al.4
Impact of ADR
Overall male patients reported a higher burden of ADRs, particularly for arthralgia, headache, and decreased drug effectiveness. Nevertheless, there was no statistically significant difference in ADR burden between female and male patients. Interestingly, pneumonia was reported by both male and female patients as being the ADR with the highest burden.
Conclusion
This Dutch study provides useful insight into the differences in ADRs experienced by male and female patients with RA, PsA, or SpA treated with etanercept or adalimumab. Female patients were significantly more likely to report ≥1 ADR than males and of larger variety. Although no significant differences were found in ADR burden between the sexes, the variety and nature of these ADRs are still informative to clinicians. Van Lint highlighted the utilization of patient-reported data using structured questionnaires, the large sample size, and the examination of ADR burden as study strengths. However, they also state that the findings were limited by uncertainty around the causal relationship of the ADRs and difficulty in making comparisons between sex-specific ADRs, such as cystitis.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

