All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The pso Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the pso Hub cannot guarantee the accuracy of translated content. The pso and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The PsOPsA Hub is an independent medical education platform, supported by educational grants. We would like to express our gratitude to the following companies for their support: UCB, for website development, launch, and ongoing maintenance; UCB, for educational content and news updates. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View psoriasis and psoriatic arthritis content recommended for you
A review of the pathogenesis and molecular targets of psoriatic disease
Do you know... Human leukocyte antigen (HLA) class I alleles are associated with hereditary psoriasis and PsA. Which of the following HLA alleles is associated with early onset psoriasis with a longer interval between skin and joint disease?
Psoriatic arthritis (PsA) may be difficult to differentiate from psoriasis owing to their overlapping phenotypes.1 Psoriatic disease or psoriatic syndrome is a term used to describe the heterogenous manifestations of PsA and psoriasis that share a common pathophysiological basis. Treatments for psoriatic disease should ideally enable a sustained and comprehensive improvement by interfering with the common underlying immune processes, thereby effecting a response across all disease manifestations. A mechanistic concept of psoriatic disease is increasingly recognized by the scientific community, which is aiding a more holistic approach in the management of the different domains of psoriatic disease.1
Here, we summarize the key aspects of this comprehensive mechanistic concept of PsA, addressing clinically relevant processes including genetic, biomechanical, metabolic, and microbial factors, that were published in a recent review by Schett et al.1 in Rheumatology.
Drivers and manifestations of psoriatic disease
Psoriatic disease involves a set of specific drivers that encourage an abnormal immune response, resulting in the development of chronic disease that requires therapeutic intervention (Figure 1).
Figure 1. Drivers, manifestations, and consequences of psoriatic disease*
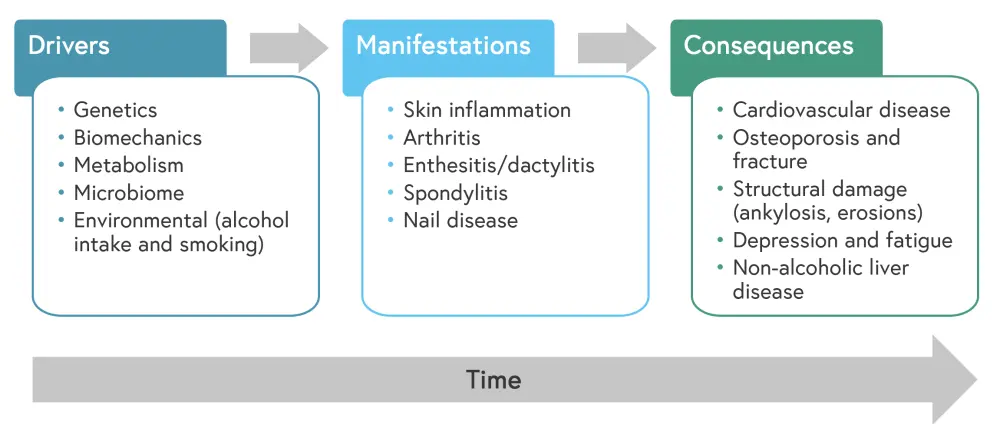
*Adapted from Schett, et al.1
Genetic factors
The contribution of genetic factors to PsA was emphasized by the 2006 Classification for Psoriatic Arthritis (CASPAR) criteria, which recommended not only considering the presence of psoriasis, but also a family history of psoriasis. More recently, data have further highlighted the role that genetic factors play in psoriatic disease, leading to an earlier onset of skin disease and a more frequent presence of enthesitis. In studies of Northern European populations, 50–90% of psoriasis was attributed to heritability. In addition, population-based studies showed a higher relative risk (30–49) of PsA among siblings, with a heritability estimate of 80–100%, suggesting that PsA may be more genetically driven than psoriasis.
HLA associations
An estimated 35–50% of the overall heritability for both psoriasis and PsA is based on human leukocyte antigen (HLA) class I alleles. The HLA allele most robustly associated with psoriatic disease is HLA-C*06; however, this association differs greatly between psoriatic skin and joint disease, suggesting these entities are genetically distinct. The association between HLA-C*06 and psoriasis is stronger than its association with PsA, and its presence correlates with earlier psoriasis onset, but a longer interval between skin and joint disease, and consequently, a milder variant of PsA.
HLA-B*27, HLA-B*39, HLA-B*38, and HLA-B*08 from the HLA-B locus are consistently associated with PsA, whereas HLA-B*44 seems to have a protective effect. A shorter interval between the onset of psoriasis and the development of inflammatory arthritis has been observed with HLA-B*27 and HLA-B*39 genotypes. HLA-B*08 has been associated with a higher prevalence of peripheral arthritis, increased joint damage, and ankylosis. HLA-B*27 is also associated with structural damage, enthesitis, dactylitis, axial disease, and symmetrical sacroiliitis. Other alleles linked with structural damage include HLA-C*01 and HLA-D*Qw3. In one study, the presence of glutamine at position 45 (Glu45) of HLA-B alleles increased the risk of psoriatic joint disease compared to skin disease. Despite the establishment of these genetic associations, HLA typing has been underutilized in clinical practice due to low risk and the limited positive and negative predictive value for each of the HLA associations. There is potential for HLA typing to be incorporated into polygenic risk scores and as part of a broader panel of tests to aid early diagnosis of PsA, as well as forecasting disease expression or prognosis.
Non-HLA associations
PSORS2 was identified, via exome sequencing, as a representative of gain-in-function mutations in caspase recruitment domain-containing protein 14 (CARD14), a protein implicated in the induction of pro-inflammatory NF-κB. A homozygous missense mutation in interleukin-36 receptor antagonists (IL36RN), which encodes IL-36 receptor antagonist (IL-36Ra), was identified in pustular psoriasis. It is thought that the deregulation of the IL-36 pathway leads to an excess of neutrophil recruitment and subsequent pustular lesions. Genotyping work has identified HLA-C, TNFRSF9, and LCE3A as being associated with psoriasis, and IL-23R, TRAF3IP2, TNFAIP3, PTPN22, and a single nucleotide polymorphism within the 5q31 susceptibility locus, have been identified as being associated with PsA.
Microbial dysbiosis
The collection of symbiotic, commensal, and pathogenic microorganisms (and their genomes), known as the microbiome, is a potential trigger for psoriatic disease. Microbial balance can be disrupted at times by several factors, including genetic, hormonal, dietary, and xenobiotic factors, leading to dysbiosis that in turn may lead to the initiation and continuation of psoriatic disease.
Microbiome and psoriatic disease
Several animal models, such as the HLA-B*27 transgenic rat and the SKG mouse, which are prone to developing psoriasiform lesions, inflammatory arthritis, and colitis, remain healthy under germ-free conditions, but go on to develop IL-23/IL-17-driven inflammatory disease when exposed to certain microbes. Studies in humans have also found perturbations in the intestinal microbiota composition in PsA and related conditions. For example, decreased levels of Akkermansia and Ruminococcus have been found in patients with PsA. These correlate with higher levels of intestinal secretory immunoglobulin A (IgA) and decreased levels of receptor activator of NF-κΒ ligand (RANKL). Similar intestinal dysbiosis has been found in patients with psoriasis. Dysbiosis can impact intestinal permeability, creating a local inflammatory state with T-cell activation and IL-23 production. Despite the increasing amount of evidence supporting the co-occurrence of intestinal inflammation and arthritis manifestations, the specific mechanism linking the gut to synovioentheseal pathogenesis is unknown.
Microbial perturbations of skin bacteria may also modulate local and distal inflammatory responses in psoriatic diseases. Studies have highlighted that bacterial and fungal taxa may be involved in the initiation of psoriasis and PsA, and others have demonstrated that commensal bacteria on the skin can affect skin immunity leading to either tolerance or the activation of cellular mediators involved in inflammatory responses.
Role of microbial metabolites
The dietary fiber-derived short-chain fatty acids (SCFAs) and medium-chain fatty acids are commonly produced microbial metabolites. SCFAs, such as butyrate, acetate, and propionate, have been shown to be involved in the expansion of regulatory T cells, as well as to abrogate immune-mediated inflammatory. This suggests that metabolic changes associated with the loss of commensal bacteria in the gut may result in a pro-inflammatory microenvironment. SCFAs also promote the differentiation of IL-10-producing T cells, suggesting they have inherent immune-regulatory characteristics. SCFAs have been found to mitigate arthritis via regulatory T cells, and to also have a regulatory effect on bone, where they inhibit osteoclasts, thereby preventing bone loss. SCFAs are dependent on diet, and a high fiber diet results in increased anti-inflammatory SCFAs, decreased levels of pro-arthritic cytokines, and restoration of microbiota.
Therapeutic implications
Several approaches have been assessed to influence psoriatic disease through modulation of the microbiome, including the use of oral antibiotics, prebiotics, and probiotics, with mixed results. A small-scale clinical trial, FLORA (NCT03058900), explored fecal microbiota transplantation (FMT) for patients with active PsA. However, the study found placebo treatment to be superior to FMT, despite amelioration of symptoms in both arms. The study was underpowered, lacked microbiome assessments, used a non-validated primary outcome, and utilized a single FMT procedure; however, it did demonstrate that the procedure was well tolerated, and the findings may enhance knowledge in PsA and host-microbial interactions.
The field of pharmacomicrobiomics aims to understand the effect microbial variations have on the action and toxicity of drugs. For example, the use of IL-17A inhibitors has been shown to result in the expansion of intestinal Candida albicans in subgroups of patients with PsA. In addition, the gut microbiome is affected differently during treatment with IL-17 or IL-23 inhibitors, which correlates with treatment response. It is important to gain a full understanding of these microbial interactions so that the various psoriasis and PsA treatments can be more precisely applied to improve clinical outcomes.
Metabolic and cardiovascular factors
Metabolic
Obesity has been identified as a risk factor for developing psoriasis; a 1-kg/m2 increase in body mass index (BMI) is associated with a 4% higher probability of psoriasis.
Adipose tissue is associated with increased expression of T cells, eosinophils, mast cells, and myeloid cells, resulting in the local release of cytokines and adipokines, such as leptin. Patients with PsA have higher concentrations of the adipokines leptin and adiponectin, which may be indicative of insulin resistance and an altered body composition, with increased central fat deposition and reduced lean mass. Interestingly, the HUNT study2 highlighted that the metabolic variations may be due to lipid changes rather than obesity in psoriatic disease onset. Free-fatty acids (FFAs) are a crucial link between dyslipidemia and inflammation, inducing cytokine expression in human macrophages, and the serum concentration of FFAs correlates with psoriasis severity in both mice and humans. In addition to the use of dietary measures to reduce body fat, tumor necrosis factor (TNF) inhibition also improves dyslipidemia and reduces adipokine concentrations.
Cardiovascular
Cardiovascular comorbidities are a well-recognized feature of psoriasis and PsA, with population-based studies suggesting an approximate 1% increase in the risk of major adverse cardiovascular events per year of psoriatic disease. High levels of cytokine and/or chemokine expression, activation of T helper cell (TH)1, TH17, and myeloid cells, upregulation of matrix metalloproteinases, and local activation of stromal cell lineages are some of the common features shared between atherosclerotic plaques and psoriatic synovial or entheseal lesions. These pathways are thought to be amplified by the systemic inflammation present in PsA. Specific cytokines, such as TNF (a potent activator of endothelial cells) and IL-17A (acts synergistically with other cytokines), may play a role in vascular damage. In addition, vascular damage may exist in the absence of conventional cardiovascular risk factors and may be associated with underlying disease severity in patients with PsA. Anti-inflammatory and TNF inhibitors have been shown to positively influence vascular risks, but further study is needed to define non-inflammatory pathways that may be involved in the metabolic pathogenesis of PsA.
Mechano-inflammation
Mechano-inflammation is the term used to describe the association of psoriatic disease with mechanical stress, and it is thought that mechano-inflammation may explain the patchy distribution of psoriatic skin and joint disease. Koebnerization, a phenomenon described over 100 years ago in which mechanical injury or trauma induces development of new psoriatic lesions, is a predictor of PsA. This phenomenon involves an overshooting of the inflammatory response to epidermal injury, which ultimately leads to dendritic cell activation and T cell recruitment to the injured skin. Once activated, dendritic cells enhance the inflammatory response and increase excessive tissue expression. The factors that specifically trigger the exaggerated response in psoriatic skin compared with normal skin require further examination.
There is a lack of evidence regarding how mechanical forces influence the onset of inflammation in PsA. Stromal cells, called tenocytes, control extracellular matrix synthesis by producing collagen or degrading it via proteases. Animal studies have demonstrated a correlation between mechanical stress and arthritis; unloading decreased mechanical stress, which inhibited arthritis, whereas running led to increased mechanical stress and increased the severity of arthritis. These effects were independent of adaptive immunity due to T cells being non-essential for increasing mechanically-induced inflammatory stromal responses. Pro-inflammatory lipid mediators, such as prostaglandin E2 (PGE2), are essential in triggering rapid vasodilation, neutrophil attraction, and angiogenesis, all of which are characteristics of PsA. In addition, PGE2 is a central nociceptive molecule that activates nociceptive neurons in dorsal root ganglia. Figure 2 shows how mechano-inflammation affects tendons in PsA.
Figure 2. Mechano-inflammation in psoriatic arthritis*
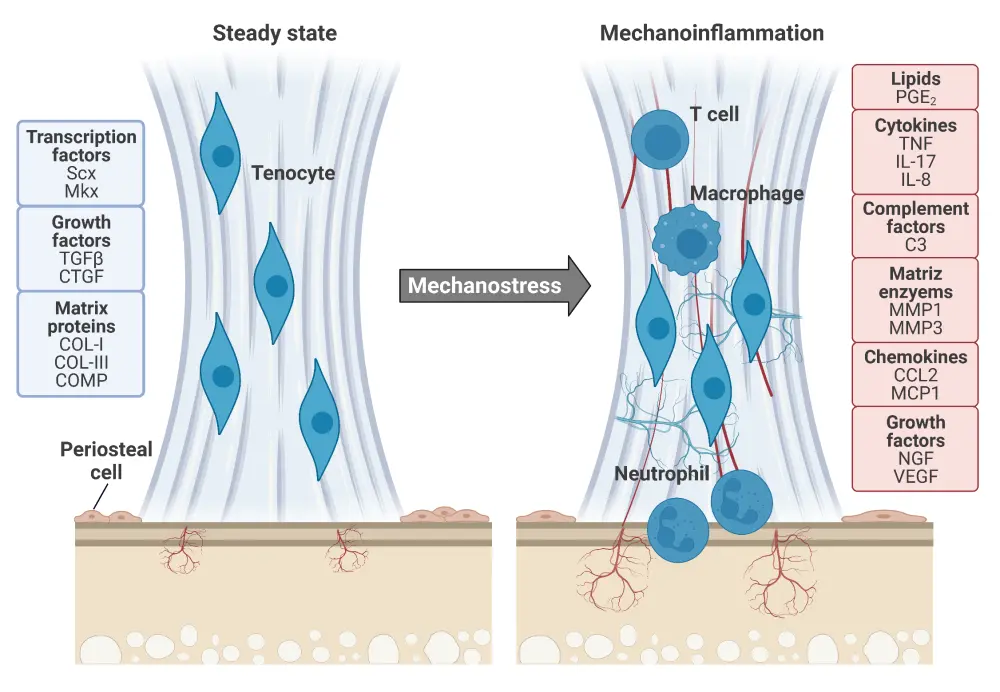
Tendons in a steady state (left) have tenocytes controlling extracellular matrix turnover using the matrix proteins, growth factors, and transcription factors listed in blue. Scx and Mkx are mechanosensitive and promote the expression of mechanical stress-activated genes. Once tendons enter an activated state after mechanostress (right), characteristic damage to collagen fibres is observed, immune cells infiltrate the tendons, and vascularization and nerve spreading lead to hyperalgesia. These alterations occur in response to the molecules listed in red.
*Adapted from Schett, et al. Created with BioRender.com.
C3, complement factor 3; CCL2, CC-chemokine ligand 2; COL, collagen; COMP, cartilage oligomeric matrix protein; CTGF, connective tissue growth factor; IL, interleukin; MCP1, monocyte chemoattractant protein-1; Mkx, mohawk; MMP, matrix metalloproteinases; NGF, nerve growth factor; PGE2, prostaglandin E2; Scx, scleraxis; TGFβ, transforming growth factor-β; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Key pro-inflammatory cytokines
Pro-inflammatory cytokines are essential in understanding the pathogenesis of PsA. Reducing inflammation and improving the signs and symptoms of PsA may be possible by inhibition of IL-23, IL-17A, and TNF. Each individual cytokine may have its own specific pathophysiological role in psoriatic disease; IL-23 as a disease initiator, IL-17A an enhancer, and TNF as a downstream effector cytokine.
IL-23 overexpression in mouse models leads to inflammation in the entheses and skin with similar features to those seen in psoriatic disease. IL-23 expression has been found to be increased in psoriasis but not in psoriatic joint disease, leading to the hypothesis that the source of IL-23 overexpression may be distal sites, such as the skin or the gut. This might lead to an enhanced circulation of IL-23 responsive cells, such as innate lymphoid cell (ILC)3 and TH17, spreading psoriatic disease to the joints.
IL-17 family members (IL-17A and IL-17F) are enhancers of inflammation in the entheses and joints of patients with PsA. IL-17A appears to be the main pro-inflammatory cytokine in PsA, with cells responsive to IL-23 being the main source of IL-17. However, alternative tissue-specific enhancements of IL-17A production can occur via the COX2 pathway, which links this cytokine production with mechano-inflammation. In addition, IL-17A stimulates neutrophil function, is known to attract neutrophils to affected tissues (skin and entheses), and has been recognized as a mediator of tendon inflammation. It is unsurprising, therefore, that inhibition of IL-17A or IL-17A/F is proving to be effective in PsA.
TNF is produced by macrophages, stimulating cytokine production, and activating fibroblasts, which leads to tissue remodeling. TNF is also produced by neutrophils and activated T cells, both of which are implicated in psoriatic disease. TNF inhibitors have been shown to have anti-inflammatory effects in PsA, likely due to the prevention of myeloid cell activation, leading TNF being seen as a downstream effector in PsA.
Structural changes in PsA
PsA is a damaging disease associated with functional decline and structural damage leading to changes in the joint and entheseal architecture (Figure 3). Entheseal lesions, areas of new bone formation at entheseal sites close to the joints, are the earliest structural change observed in PsA, are often present in patients with psoriasis even before PsA develops, and are closely associated with functional impairment. Inflammation at entheseal sites triggers mesenchymal cells to proliferate and differentiate into bone-forming osteoblasts. Ongoing growth of structural entheseal lesions leads to the formation of bony spurs or enthesiophytes and are responsible for shaping the characteristic clinical presentation of PsA.
Another important structural change is bone erosion, which is also common in rheumatoid arthritis. Erosions typically occur after synovitis as a result of the formation of osteoclasts in the synovial microenvironment (Figure 3). Trabecular bone loss has also been observed early in psoriatic disease, with mild bone loss already present in patients with psoriasis, particularly in those with high cytokine concentrations. Patients with PsA treated with biologic disease-modifying antirheumatic drugs show reduced bone loss compared with conventionally treated patients.
Figure 3. Structural changes in psoriatic arthritis*
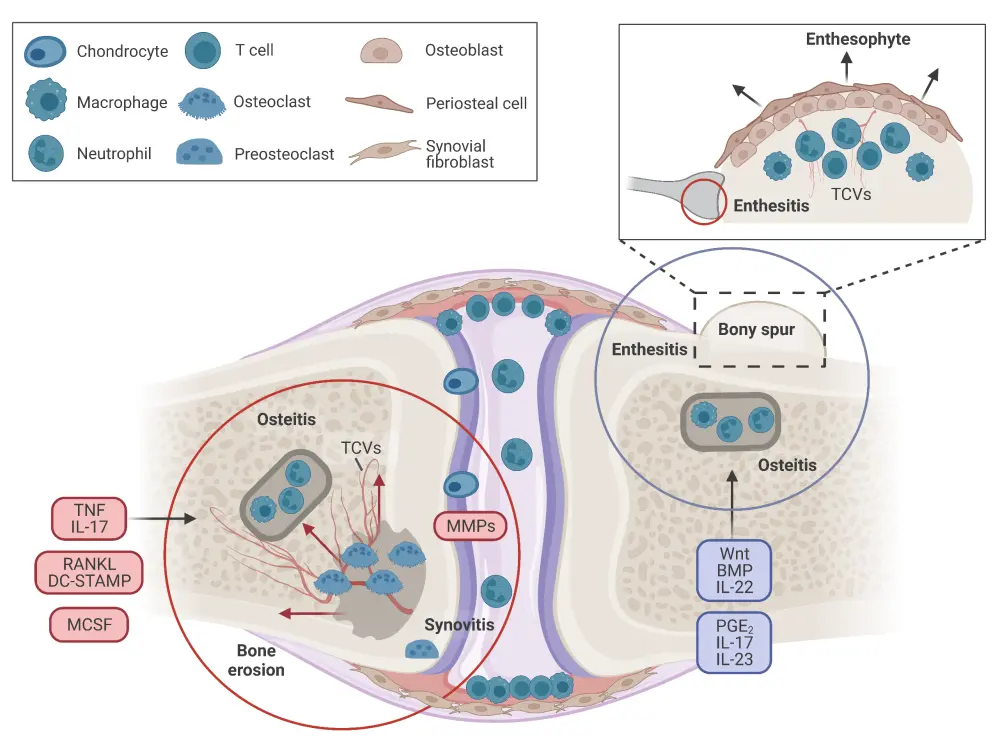
Both bone erosion (left circle) and enthesophyte formation (right circle) processes are responsible for the characteristic structural changes seen in psoriatic arthritis. Bone erosion is an intra-articular process whereby synovial inflammation leads to the differentiation of both pre-osteoclasts and osteoclasts resulting in bone resorption. Enthesitis, an extra-articular process, occurs via periosteal activation and osteoblast differentiation, leading to matrix deposition and bone formation. TCVs connect synovial inflammation and enthesitis with bone marrow inflammation.
*Adapted from Schett, et al. Created with BioRender.com.
BMP, bone morphogenic protein; DC-STAMP, dendritic cell-specific transmembrane protein; IL, interleukin; MCSF, macrophage-colony-stimulating factor; MMP, matrix metalloproteinase; PGE2, prostaglandin E2; RANKL, receptor activator of nuclear factor κB ligand; TCV, transcortical vessel; TNF, tumor necrosis factor.
Drivers of psoriatic disease and its management
Taken together, these mechanisms provide an insight into the molecular pathogenesis of psoriatic disease and can be used to appropriately manage psoriatic disease (Table 1).
Table 1. Drivers of psoriatic disease and related interventions*
|
IL, interleukin; JAK, Janus kinase; NSAID, non-steroidal anti-inflammatory drug; PDE4, phosphodiesterase 4; TNF, tumor necrosis factor. |
|
|
Driver mechanism |
Intervention |
|---|---|
|
Metabolism |
Weight loss |
|
Mechano-inflammation |
Muscle strengthening and stretching regularly |
|
Intestinal dysbiosis |
Fiber-rich diet |
|
IL-23 (inducer cytokine) |
Guselkumab |
|
IL-17A (enhancer cytokine) |
Secukinumab |
|
TNF (effector cytokine) |
Adalimumab |
|
Intracellular enzymes and/or kinases |
Apremilast (PDE4) |
|
Environmental (alcohol and smoking) |
Reduction and cessation of alcohol and smoking |
Conclusion
This review highlights that psoriatic disease is complex and multifactorial, with common pathogenic processes, including genetic susceptibility, altered response to mechanical stress, microbial dysbiosis, and metabolic disturbances. Inhibition of TNF, IL-17, and IL-23 has been found to be effective in musculoskeletal disease, but tissue-specific exceptions exist, such as the prevalence of IL‑17 and IL-23 in skin disease, and differences in their inhibition in intestinal disease. This highlights the need for further exploration into whether inhibition using a combination of treatments might offer more profound response rates or lead to an increase in treatment-related adverse events. In addition, the drivers of psoriatic disease must be managed appropriately using a range of interventions. Early diagnosis is vital for the prevention of functional and structural damage in the absence of a cure for psoriatic disease.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with plaque psoriasis do you see per month?

